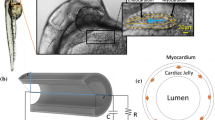
Abstract
In this paper, we develop a neuromechanical model of pumping in a valveless, tubular heart inspired by the tunicate, Ciona savignyi. Valveless, tubular hearts are common throughout the animal kingdom. The vertebrate embryonic heart first forms as a valveless, tubular pump. The embryonic, juvenile, and adult hearts of many invertebrates are also valveless, tubular pumps. Several different pumping mechanisms have been propsed for tubular hearts, and it is not clear if all animals employ the same mechanism. We compare the flows generated by this pumping mechanisms to those produced by peristalsis using a prescribed contraction wave and to those produced by impedance pumping across a parameter space relevant to Ciona savignyi. The immersed boundary method is used to solve the fully-coupled fluid-structure interaction problem of an elastic tubular heart immersed in a viscous fluid. The FitzHugh–Nagumo equations are used to model the propagation of the action potential which initiates the contraction. We find that for the scales relevant to Ciona, both the neuromechanical pumping mechanism and peristalsis produce the strong flows observed in the tunicate heart. Only the neuromechanical model produces flow patterns with all of the characteristics reported for valveless, tubular hearts. Namely, the neuromechanical pump generates a bidirectional wave of contraction and peristalsis does not.









Similar content being viewed by others
References
Anderson, M.: Electrophysiological studies on initiation and reversal of the heart beat in Ciona intestinalis. J. Exp. Biol. 49, 363–385 (1968)
Baird, A., King, T., Miller, L.A.: Numerical study of scaling effects in peristalsis and dynamic suction pumping. In: Proceedings of the AMS, Special Session on Biological Fluid Dynamics: Modeling, Computations, and Applications, vol. 628, pp. 129–148 (2014)
Bringley, T., Childress, S., Vandenberghe, N., Zhang, J.: An experimental investigation and a simple model of a valveless pump. Phys. Fluids 20(033), 602 (2008)
Davidson, B.: Ciona intestinalis as a model for cardiac development. Semin. Cell Dev. Biol. 18, 16–26 (2007)
FitzHugh, R.: Impulses and physiological states in theoretical models of nerve membrane. Biophys. J. 1(6), 445–466 (1961)
Forouhar, A.S., Liebling, M., Hickerson, A., Nasiraei-Moghaddam, A., Tsai, H., Hove, J.R., Fraser, S.E., Dickinson, M.E., Gharib, M.: The embryonic vertebrate heart tube is a dynamic suction pump. Science 312(5774), 751–753 (2006). doi:10.1126/science.1123775. http://www.sciencemag.org/content/312/5774/751.abstract. http://www.sciencemag.org/content/312/5774/751.full.pdf
Harrison, J., Waters, J., Cease, A., Cease, A., VandenBrooks, J., Callier, V., Klok, C., Shaffer, K., Socha, J.: How locusts breathe. Physiology 28, 18–27 (2013)
Hickerson, A.I., Rinderknecht, D., Gharib, M.: Experimental study of the behavior of a valveless impedance pump. Exp. Fluids 38(4), 534–540 (2005)
Hodgkin, A.L., Huxley, A.F.: Propagation of electrical signals along giant nerve fibres. In: Proceedings of the Royal Society of London Series B, Biological Sciences, pp. 177–183 (1952)
Jung, E., Peskin, C.: Two-dimensional simulations of valveless pumping using the immersed boundary method. SIAM J. Sci. Comput. 23(1), 19–45 (2001). doi:10.1137/S1064827500366094. http://epubs.siam.org/doi/abs/10.1137/S1064827500366094. http://epubs.siam.org/doi/pdf/10.1137/S1064827500366094
Kalk, M.: The organization of a tunicate heart. Tissue Cell 2, 99–118 (1970)
Keener, J.P.: Wave propagation in Myocardium. In: Glass, L., Hunter, P., McCulloch, A. (eds.) Theory of Heart –Biomechanics, Biophysics, and Nonlinear Dynamics of Cardiac Function, pp. 405–436. Springer, New York (1991)
Kriebel, M.E.: Conduction velocity and intracellular action potentials of the tunicate heart. J. Gen. Physiol. 50(8), 2097–2107 (1967)
Lemaire, P.: Evolutionary crossroads in developmental biology: the tunicates. Development 138, 2143–2152 (2011)
Liebau, G.: Über ein ventilloses pumpprinzip. Naturwissenschaften 41, 327–327 (1954). doi:10.1007/BF00644490
Liebau, G.: Die stromungsprinzipien des herzens. Z Kreislaufforsch 44, 677 (1955)
Männer, J., Wessel, A., Yelbuz, T.: How does the tubular embryonic heart work? looking for the physical mechanism generating unidirectional blood flow in the valveless embryonic heart tube. Dev. Dyn. 239, 1035–1046 (2010)
McMahon, B., Wilkens, J., Smith, P.: Invertebrate circulatory systems. Compr. Physiol. Suppl. 30, 931–1008 (2011)
Miller, L.A., Peskin, C.S.: Flexible clap and fling in tiny insect flight. J. Exp. Biol. 212(19), 3076–3090 (2009)
Mittal, R.: Locomotion with flexible propulsors: Ii. Computational modeling of pectoral fin swimming in sunfish. Bioinspir. Biomim. 1, S35–S41 (2006)
Nichols, D.: The water-vascular system in living and fossil echinoderms. Palaeontology 15(4), 519–538 (1972)
Pendar, H., Kenny, M.C., Socha, J.J.: Tracheal compression in pupae of the beetle zophobas morio. Biol. Lett. 11(6), 20150,259 (2015)
Peskin, C.S.: The immersed boundary method. Acta Numer. 11, 479–517 (2002). doi:10.1017/S0962492902000077. http://journals.cambridge.org/article_S0962492902000077
Peskin, C.S., McQueen, D.M.: Fluid dynamics of the heart and its valves. In: Othmer, H.G., Adler, F.R., Lewis, M.A., Dallon, J.C. (eds.) Case Studies in Mathematical Modeling: Ecology, Physiology, and Cell Biology, 2nd edn. Prentice-Hall, New Jersey (1996)
Pozrikidis, C.: A study of peristaltic flow. J. Fluid Mech. 180, 515–527 (1987)
Socha, J.J., Lee, W.K., Harrison, J.F., Waters, J.S., Fezzaa, K., Westneat, M.W.: Correlated patterns of tracheal compression and convective gas exchange in a carabid beetle. J. Exp. Biol. 211(21), 3409–3420 (2008)
Teran, J., Fauci, L., Shelley, M.: Viscoelastic fluid response can increase the speed and efficiency of a free swimmer. Phys. Rev. Lett. 104(3), 38,101 (2010)
Tytell, E.D., Hsu, C., Williams, T.L., Cohen, A.H., Fauci, L.J.: Interactions between internal forces, body stiffness, and fluid environment in a neuromechanical model of lamprey swimming. Proc. Natl. Acad. Sci. 107(46), 19,832–19,837 (2010)
Waldrop, L., Miller, L.: The role of the pericardium in the valveless, tubular heart of the tunicate, ciona savignyi. J. Exp. Biol. 218, 2753–2763 (2015a). doi:10.1242/jeb.116863
Waldrop, L., Miller, L.A.: Large-amplitude, short-wave peristalsis and its implications for transport. Biomech. Model. Mechanobiol. (2015b). doi:10.1007/s10237-015-0713
Xavier-Neto, J., Castro, R., Sampaio, A., Azambuja, A., Castillo, H., Cravo, R., Simoes-Costa, M.: Parallel avenues in the evolution of hearts and pumping organs. Cell. Mol. Life Sci. 64, 719–734 (2007)
Xavier-Neto, J., Davidson, B., Simoes-Costa, M., Castillo, H., Sampaio, A., Azambuja, A.: Evolutionary origins of the heart. In: Rosenthal, N., Harvey, R. (eds.) Heart Development and Regeneration, vol. 1, 1st edn, pp. 3–38. Elsevier Science and Technology, London (2010)
Acknowledgments
The authors would like to thank the Japanese Society for Mathematical Biology and the Society of Mathematical Biology for their support to attend the conference. This work was funded by NSF DMS CAREER # 1151478 awarded to L. A. M. and NSF DMS RTG # 0943851 to R. McLaughlin.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Baird, A., Waldrop, L. & Miller, L. Neuromechanical pumping: boundary flexibility and traveling depolarization waves drive flow within valveless, tubular hearts. Japan J. Indust. Appl. Math. 32, 829–846 (2015). https://doi.org/10.1007/s13160-015-0195-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13160-015-0195-3
Keywords
- Tubular hearts
- Peristalsis
- Liebau pumping
- Immersed boundary method
- FitzHugh–Nagumo
- Embryonic hearts
- Tunicates