Abstract
Background
Previous studies showed altered angiopoietin-like protein-8 (ANGPTL-8) circulating levels in type 2 diabetes mellitus (DM). Whether or not the alteration in ANGPTL-8 level can be a predictive maker for increased DM risk remains unclear.
Aim
Investigating possible role of ANGPTL-8 as a risk predictor of type2 DM, in addition to a set of factors likely to affect ANGPTL-8 level.
Methods
One hundred recently diagnosed persons with type 2 DM and 100 sex- and age-matched healthy controls were enrolled. Exclusion criteria included type 1 DM, acute infections, history of chronic kidney disease, malignancy, and blood loss or transfusion. Serum levels of ANGPTL-8, blood pressure, weight, height, glycosylated hemoglobin (HbA1c), fasting blood glucose, cystatin C, lipid profile, liver, and kidney function tests were assessed. The independent relationship between DM and ANGPTL-8 was tested in the unadjusted and multiple-adjusted regression models.
Results
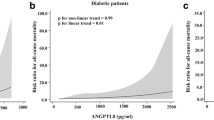
Serum ANGPTL-8 levels showed significant elevation among persons with vs. without DM (p = 0.006), positive correlation with HbA1c (p < 0.001), and negative correlation with estimated GFR (eGFR) (p = 0.003) but no significant correlation to fasting glucose level. In the unadjusted model, patients in the third tertile of ANGPTL-8 had 4 times risk of DM (OR 4.03; 95% CI = 1.37–11.84). Data adjustment for cardiovascular diseases, smoking, body mass index, systolic blood pressure, alanine transaminase (ALT), and low-density lipoprotein (LDL) increased the direct relationship between ANGPTL-8 and DM (OR 6.26; 95% CI = 1.21–32.50). However, the risk significantly decreased after adjustment of Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eGFR creatinine-cystatin (OR 2.17; 95% CI = 0.10–49.84).
Conclusion
This study highlights a possible predictive role of ANGPTL-8 in diabetic complications, particularly nephropathy. Larger prognostic studies are needed to validate the cause-effect relationship between ANGPTL-8 and deteriorated kidney functions.

Similar content being viewed by others
References
Duarte AA, Mohsin S, Golubnitschaja O. Diabetes care in figures: current pitfalls and future scenario. EPMA J. 2018;9(2):125–31.
International diabetes federation. Diabetes atlas, 8th edn. 2017. Available at : www.diabetesatlas.org. Accessed 15 June 2019.
Golubnitschaja O, Baban B, Boniolo G, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23.
Golubnitschaja O. Advanced diabetes care: three levels of prediction, prevention & personalized treatment. Curr Diabetes Rev. 2010;6(1):42–51.
Suwannaphant K, Laohasiriwong W, Puttanapong N, et al. Association between socioeconomic status and diabetes mellitus: the National Socioeconomics Survey, 2010 and 2012. J Clin Diagn Res. 2017;11(7):LC18–22.
Ge S, Xu X, Zhang J, et al. Suboptimal health status as an independent risk factor for type 2 diabetes mellitus in a community-based cohort: the China suboptimal health cohort study. EPMA J. 2019;10(1):65–72.
Soliman N, El-Shabrawi M, Omar S. DNA fragmentation damage as a predictive marker for diabetic nephropathy in type II diabetes mellitus. J Endocrinol Metab Diabetes S Afr. 2018;23(2):32–5.
Saadeldin MK, Elshaer SS, Emara IA, et al. Serum sclerostin and irisin as predictive markers for atherosclerosis in Egyptian type II diabetic female patients: a case control study. PLoS One. 2018;13(11):e0206761.
Lee H, Park T, Kim B. Metabolic markers predictive of prediabetes in the Korean population. Diabetes. 2018;67(Supplement 1). https://doi.org/10.2337/db18-201-LB.
Golubnitschaja O, Costigliola V. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3(1):14.
Gupta M, Singh JP. Correlation of microalbuminuria with glycosylated haemoglobin in patients of diabetes having nephropathy. Int J Adv Med. 2017;4(3):805–8.
Sena CM, Bento CF, Pereira P, et al. Diabetes mellitus: new challenges and innovative therapies. EPMA J. 2010;1(1):138–63.
Golubnitschaja O, Costigliola V. EPMA summit 2014 under the auspices of the presidency of Italy in the EU: professional statements. EPMA J. 2015;6(1):4.
Ren G, Kim JY, Smas CM. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am J Physiol Endocrinol Metab. 2012;303(3):E334–51.
Quagliarini F, Wang Y, Kozlitina J, et al. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci U S A. 2012;109(48):19751–6.
Zhang R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem Biophys Res Commun. 2012;424(4):786–92.
Fu Z, Yao F, Abou-Samra AB, et al. Lipasin, thermoregulated in brown fat, is a novel but atypical member of the angiopoietin-like protein family. Biochem Biophys Res Commun. 2013;430(3):1126–31.
Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic beta cell proliferation. Cell. 2013;153(4):747–58.
Luo M, Peng D. ANGPTL8: an important regulator in metabolic disorders. Front Endocrinol (Lausanne). 2018;9:169.
Maurer L, Schwarz F, Fischer-Rosinsky A, et al. Renal function is independently associated with circulating betatrophin. PLoS One. 2017;12(3):e0173197.
Zhang R, Abou-Samra AB. Emerging roles of lipasin as a critical lipid regulator. Biochem Biophys Res Commun. 2013;432(3):401–5.
Yi P, Park JS, Melton DA. Retraction notice to: betatrophin: a hormone that controls pancreatic beta cell proliferation. Cell. 2017;168(1–2):326.
Li S, Liu D, Li L, et al. Circulating betatrophin in patients with type 2 diabetes: a meta-analysis. J Diabetes Res. 2016;2016:6194750.
Leiherer A, Muendlein A, Geiger K, et al. Betatrophin is associated with type 2 diabetes and markers of insulin resistance. Diabetes. 2018;67(Supplement 1). https://doi.org/10.2337/db18-2445-PUB.
Gomez-Ambrosi J, Pascual E, Catalan V, et al. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J Clin Endocrinol Metab. 2014;99(10):E2004–9.
Gokulakrishnan K, Manokaran K, Pandey GK, et al. Relationship of betatrophin with youth onset type 2 diabetes among Asian Indians. Diabetes Res Clin Pract. 2015;109(1):71–6.
Fenzl A, Itariu BK, Kosi L, et al. Circulating betatrophin correlates with atherogenic lipid profiles but not with glucose and insulin levels in insulin-resistant individuals. Diabetologia. 2014;57(6):1204–8.
Espes D, Lau J, Carlsson PO. Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes. Diabetologia. 2014;57(1):50–3.
Fu Z, Berhane F, Fite A, et al. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci Rep. 2014;4:5013.
Zhang R. Abou-Samra AB. A dual role of lipasin (betatrophin) in lipid metabolism and glucose homeostasis: consensus and controversy. Cardiovasc Diabetol. 2014;13:133.
Hu H, Sun W, Yu S, et al. Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care. 2014;37(10):2718–22.
Tuhan H, Abaci A, Anik A, et al. Circulating betatrophin concentration is negatively correlated with insulin resistance in obese children and adolescents. Diabetes Res Clin Pract. 2016;114:37–42.
Battal F, Turkon H, Aylanc N, et al. Investigation of blood Betatrophin levels in obese children with non-alcoholic fatty liver disease. Pediatr Gastroenterol Hepatol Nutr. 2018;21(2):111–7.
Ebert T, Kralisch S, Hoffmann A, et al. Circulating angiopoietin-like protein 8 is independently associated with fasting plasma glucose and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2014;99(12):E2510–7.
Chen CC, Susanto H, Chuang WH, et al. Higher serum betatrophin level in type 2 diabetes subjects is associated with urinary albumin excretion and renal function. Cardiovasc Diabetol. 2016;15:3.
Cioms W. International ethical guidelines for epidemiological studies. Geneva. 2009.
American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;39(Suppl 1):S13–22.
Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2013;367(1):20–9.
Grambsch PM, Therneau TM, Fleming TR. Diagnostic plots to reveal functional form for covariates in multiplicative intensity models. Biometrics. 1995;51(4):1469–82.
Pan R, Zhang H, Yu S, et al. Betatrophin for diagnosis and prognosis of mothers with gestational diabetes mellitus. J Int Med Res. 2019;47(2):710–7.
Abu-Farha M, Abubaker J, Al-Khairi I, et al. Higher plasma betatrophin/ANGPTL8 level in type 2 diabetes subjects does not correlate with blood glucose or insulin resistance. Sci Rep. 2015;5:10949.
Lee SH, Rhee M, Kwon HS, et al. Serum betatrophin concentrations and the risk of incident diabetes: a nested case-control study from Chungju metabolic disease cohort. Diabetes Metab J. 2018;42(1):53–62.
Fortwaengler K, Parkin CG, Neeser K, et al. Description of a new predictive modeling approach that correlates the risk and associated cost of well-defined diabetes-related complications with changes in glycated hemoglobin (HbA1c). J Diabetes Sci Technol. 2017;11(2):315–23.
Penno G, Solini A, Bonora E, et al. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care. 2013;36(8):2301–10.
Guo K, Lu J, Yu H, et al. Serum betatrophin concentrations are significantly increased in overweight but not in obese or type 2 diabetic individuals. Obesity (Silver Spring). 2015;23(4):793–7.
Yamada H, Saito T, Aoki A, et al. Circulating betatrophin is elevated in patients with type 1 and type 2 diabetes. Endocr J. 2015;62(5):417–21.
Ebert T, Kralisch S, Wurst U, et al. Betatrophin levels are increased in women with gestational diabetes mellitus compared to healthy pregnant controls. Eur J Endocrinol. 2015;173(1):1–7.
Mahmood D, Makoveichuk E, Nilsson S, et al. Response of angiopoietin-like proteins 3 and 4 to hemodialysis. Int J Artif Organs. 2014;37(1):13–20.
Pfau D, Bachmann A, Lossner U, et al. Serum levels of the adipokine chemerin in relation to renal function. Diabetes Care. 2010;33(1):171–3.
Stein S, Bachmann A, Lossner U, et al. Serum levels of the adipokine FGF21 depend on renal function. Diabetes Care. 2009;32(1):126–8.
Merabet E, Dagogo-Jack S, Coyne DW, et al. Increased plasma leptin concentration in end-stage renal disease. J Clin Endocrinol Metab. 1997;82(3):847–50.
Golubnitschaja O. Time for new guidelines in advanced diabetes care: paradigm change from delayed interventional approach to predictive, preventive & personalized medicine. EPMA J. 2010;1(1):3–12.
Acknowledgments
The authors would like to acknowledge the efforts of the medical postgraduate student Mohamed Abd Allah ElKelany, in data collection and entry.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception of idea, study design, laboratory investigations, interpretation of results, writing and revising the manuscript, providing intellectual content of critical importance to the work described, and final approval of the version to be published. In addition, Dr. Yasmine Amr Issa carried out all laboratory investigations, Dr. Samar Samy Abd ElHafeez was responsible for statistical analysis, and Dr. Noha Gaber Amin executed the recruitment, examination, and data collection of patients. All authors are also accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval for human studies
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Alexandria Faculty of Medicine Ethics of the research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Issa, Y.A., Abd ElHafeez, S.S. & Amin, N.G. The potential role of angiopoietin-like protein-8 in type 2 diabetes mellitus: a possibility for predictive diagnosis and targeted preventive measures?. EPMA Journal 10, 239–248 (2019). https://doi.org/10.1007/s13167-019-00180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-019-00180-3