Abstract
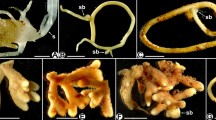
We provide a preliminary report of the mycobionts found within four Monotropoideae (Ericaceae) species from China: Monotropa uniflora, Hypopitys monotropa, Monotropastrum humile and Monotropastrum sciaphilum (a rare endemic species never previously studied for mycorrhizae). Such achlorophyllous Monotropoideae plants obtain their carbohydrates from mycorrhizal fungi linking them to surrounding trees, on which these fungi form ectomycorrhizae. Since Monotropoideae were rarely studied in continental Asia, the root systems of the four species sampled in Yunnan were examined using morphological and molecular methods. All the roots of these four species exhibit a typical monotropoid mycorrhizal morphology, including a fungal mantle, a Hartig net and hyphal pegs. In M. uniflora and M. humile mycorrhizae, cystidia typical of Russula symbionts covered the fungal mantle. ITS barcoding revealed that Russulales were the most frequent colonizers in all species, but Hypopitys monotropa displayed various additional mycorrhizal taxa. Moreover, a few additional ectomycorrhizal and saprotrophic Basidiomycota taxa were identified in the three other species, challenging that these four Monotropoideae species are as strictly fungal specific as the other Monotropoideae species hitherto studied. Moreover, a comparison with accompanying fungus sporocarps revealed that the fruiting fungal community significantly differed from that associated with the Monotropoideae roots, so that a clear fungal preference was evident. Finally, four fungal species were found on more than one Monotropoideae species: this contrasted with previous reports of sympatrically growing mycoheterotrophic plants, which did not reveal any overlap. This again challenges the idea of strict fungal specificity.






Similar content being viewed by others
References
Beatty GE, Provan J (2011) Phylogeographic analysis of North American populations of the parasitic herbaceous plant Monotropa hypopitys L. reveals a complex history of range expansion from multiple late glacial refugia. J Biogeogr 38:1585–1599
Beenken L (2004) Les ectomycorhizes du genre Russula. Bull Soc Mycol Fr 120:293–333
Bidartondo MI, Bruns TD (2001) Extreme specificity in epiparasitic Monotropoideae (Ericaceae): widespread phylogenetic and geographical structure. Mol Ecol 10:2285–2295
Bidartondo MI, Bruns TD (2002) Fine-level mycorrhizal specificity in the Monotropoideae (Ericaceae): specificity for fungal species groups. Mol Ecol 11:557–569
Bidartondo MI, Bruns TD (2005) On the origins of extreme mycorrhizal specificity in the Monotropoideae (Ericaceae): performance trade-offs during seed germination and seedling development. Mol Ecol 14:1549–1560
Björkman E (1960) Monotropa hypopithys L.—an epiparasite on tree roots. Plant Physiol 13:308–327
Bridge PD, Schlitt T, Cannon PF, Buddie AG, Baker M, Borman AM (2008) Domain II hairpin structure in ITS1 sequence as an aid in differentiating recently evolved animal and plant pathogenic fungi. Mycopathologia 166:1–16
Buyck B, Hofstetter V, Eberhardt U, Verbeken A, Kauff F (2008) Walking the thin line between Russula and Lactarius: the dilemma of Russula subsect. Ochricompactae. Fungal Divers 28:15–40
Buyck B, Hofstetter V, Verbeken A, Walleyn R (2010) Prop. 1919: To conserve Lactarius nom. cons. (Basidiomycota) with a conserved type. Mycotaxon 111:504–508
Cullings K, Szaro T, Bruns T (1996) Evolution of extreme specialization within a lineage of ectomycorrhizal epiparasites. Nature 379:63–66
Dexheimer J, Gérard J (1993) Application de quelques techniques cytochimiques à l’étude des interfaces des ectendomycorhizes de Monotrope (Monotropa hypopitys L.). Acta Bot Gall 140:459–472
Douhan GW, Vincenot L, Gryta H, Selosse M-A (2011) Population genetics of ectomycorrhizal fungi: from current knowledge to emerging directions. Fungal Biol 115:569–597
Dowie NJ, Hemenway JJ, Trowbridge SM, Miller SL (2011) Mycobiont overlap between two mycoheterotrophic genera of Monotropoideae (Pterospora andromedea and Sarcodes sanguinea) found in the Greater Yellowstone Ecosystem. Symbiosis 54:29–36
Duddridge JA (1985) A comparative ultrastructural analysis of the host-fungus interface in mycorrhizal and parasitic association. In: Moore D, Casselton LA, Wood DA, Frankland JC (eds) Developmental biology of higher fungi. (British Mycology Society Symposium 10) Cambridge University Press, Cambridge
Duddridge JA, Read DJ (1982) An ultrastructural analysis of the development of mycorrhizas in Monotropa hypopitys L. New Phytol 92:203–214
Fang M, Fang R, Fang RC, He M, Hu L, Yang H, Qin H, Min T, Chamberlain DC, Stevens PF, Wallace GD, Anderberg A (2005) Ericaceae. In: Wu Z, Raven P, Hong D (eds) Flora of China volume 14 (Apiaceae through Ericaceae). Science Press, Beijin, pp 242–517
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—applications to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gardes M, Bruns TD (1996) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above- and below-ground views. Can J Bot 74:1572–1583
Hashimoto Y, Fukukawa S, Kunishi A, Suga H, Richard F, Sauve M, Selosse M-A (2012) Mycoheterotrophic germination of Pyrola asarifolia dust seeds reveals convergences with germination in orchids. New Phytol in press
Higgins D, Thompson J, Gibson T, Thompson JD, Higgins G, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Hughes KW, Petersen RH, Lickey EB (2009) Using heterozygosity to estimate a percentage DNA sequence similarity for environmental species’ delimitation across basidiomycete fungi. New Phytol 182:795–798
Hynson NA, Bruns TD (2009) Evidence of a myco-heterotroph in the plant family Ericaceae that lacks mycorrhizal specificity. Proc Roy Soc B 276:4053–4059
Klooster MR, Culley TM (2010) Population genetic structure of the mycoheterotroph Monotropa hypopitys L. (Ericaceae) and differentiation between red and yellow color forms. Int J Plant Sci 171:167–174
Kron KA, Judd WS, Stevens PF, Crayn DM, Anderberg AA, Gadek PA, Quinn CJ, Luteyn JL (2002) Phylogenetic classification of the Ericaceae: molecular and morphological evidence. Bot Rev 68:335–424
Leake JR (1994) The biology of myco-heterotrophic (“saprophytic”) plants. New Phytol 127:171–216
Leake JR, McKendrick SL, Bidartondo M, Read DJ (2004) Symbiotic germination and development of the myco-heterotroph Monotropa hypopitys in nature and its requirement for locally distributed Tricholoma spp. New Phytol 163:405–423
Martin JF (1985) Sur la mycorhization de Monotropa hypopitys par quelques espèces du genre Tricholoma. Bull Soc Mycol France 101:249–256
Martin JF (1986) Mycorhization de Monotropa uniflora L. par des Russulaceae. Bull Soc Mycol France 102:155–159
Martos F, Dulormne M, Pailler T, Bonfante P, Faccio A, Fournel J, Dubois M-P, Selosse M-A (2009) Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytol 184:668–681
Massicotte HB, Melville LH, Peterson RL (2005) Structural features of mycorrhizal associations in two members of the Monotropoideae, Monotropa uniflora and Pterospora andromedea. Mycorrhiza 15:101–110
Massicotte HB, Melville LH, Peterson RL, Tackaberry LE, Luoma DL (2010) Structural characteristics of root–fungus associations in two mycoheterotrophic species, Allotropa virgata and Pleuricospora fimbriolata (Monotropoideae), from southwest Oregon, USA. Mycorrhiza 20:391–397
Matsuda Y, Yamada A (2003) Mycorrhizal morphology of Monotropastrum humile collected from six different forests in central Japan. Mycologia 95:993–997
Matsuda Y, Okochi S, Katayama T, Yamada A, Ito S-I (2011) Mycorrhizal fungi associated with Monotropastrum humile (Ericaceae) in central Japan. Mycorrhiza 21:569–576
Miller SL, Buyck B (2002) Molecular phylogeny of the genus Russula in Europe with a comparison of modern infrageneric classifications. Mycol Res 106:259–276
Min S, Chang-Qin Z, Wallace GD (2011) Rediscovery of Monotropastrum sciaphilum (Andres) G. D. Wallace in China after 91 years. Aliso 29:115–118
Robertson DC, Robertson JA (1982) Ultrastructure of Pterospora andromedea Nuttall and Sarcodes sanguinea Torrey mycorrhizas. New Phytol 92:539–551
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Roy M, Watthana S, Stier A, Richard F, Vessabutr S, Selosse MA (2009) Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biol 7:51
Selosse M-A, Roy M (2009) Green plants eating fungi: facts and questions about mixotrophy. Trends Plant Sci 14:64–70
Selosse M-A, Weiss M, Jany JL, Tillier A (2002) Communities and populations of sebacinoid basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis and neighbouring tree ectomycorrhizae. Mol Ecol 11:1831–1844
Selosse M-A, Richard F, He X, Simard S (2006) Mycorrhizal networks: les liaisons dangereuses. Trends Ecol Evol 11:621–628
Selosse M-A, Martos F, Perry B, Maj P, Roy M, Pailler T (2010) Saprotrophic fungal symbionts in tropical achlorophyllous orchids: Finding treasures among the ‘molecular scraps’? Plant Signal Behav 5:1–5
Selosse M-A, Boullard B, Richardson D (2011) Noël Bernard (1874–1911): orchids to symbiosis in a dozen years, one century ago. Symbiosis 54:61–68
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Snetselaar KM, Whitney KD (1990) Fungal calcium oxalate in mycorrhizae of Monotropa uniflora. Can J Bot 68:533–543
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Taylor DL, Bruns TD (1997) Independent, specialized invasions of the ectomycorrhizal mutualism by two non-photosynthetic orchids. PNAS 94:4510–4515
Tsukaya H, Yokoyama J, Imaichi R, Ohba H (2008) Taxonomic status of Monotropastrum humile, with special reference to M. humile var. glaberrimum (Ericaceae, Monotropoideae). J Plant Res 121:271–278
Tucker G (2009) Ericaceae. In: Flora of North America Editorial Committee (ed) Flora of North America, volume 8. Oxford University Press, New-York, pp 370–535
United States Department of Agriculture (1993) Forest ecosystem management: an ecological, economic, and social assessment. A report of the Forest Ecosystem Management Assessment Team. U.S. Department of Agriculture/U.S. Department of the Interior/National Oceanic and Atmospheric Administration/U.S. Environmental Protection Agency, Portland (Oregon)
Vasiliauskas R, Menkis A, Finlay RD, Stenlid J (2007) Wood-decay fungi in fine living roots of conifer seedlings. New Phytol 174:441–446
Verkeben A, Nuytinck J, Buyck B (2012) New combinations in Lactifluus. 1. L. subgenera Edules, Lactariopsis, and Russulopsis. Mycotaxon 118:447–453
Wallace GD (1987) Transfer of Eremotropa sciaphila to Monotropastrum (Ericaceae: Monotropoideae). Taxon 36:128–130
Yamada A, Kitamura D, Setoguchi M, Matsuda Y, Hashimoto Y, Matsushita N, Fukuda M (2008) Monotropastrum humile var. humile is associated with diverse ectomycorrhizal Russulaceae fungi in Japanese forests. Ecol Res 23:983–993
Yang S, Pfister DH (2006) Monotropa uniflora plants of eastern Massachusetts form mycorrhizae with a diversity of russulacean fungi. Mycologia 98:535–540
Yokoyama J, Fukuda T, Tsukaya H (2005) Molecular identification of the mycorrhizal fungi of the epiparasitic plant Monotropastrum humile var. glaberrimum (Ericaceae). J Plant Res 118:53–56
Young BW, Massicotte HB, Tackaberry LE, Baldwin QF, Egger KN (2002) Monotropa uniflora: morphological and molecular assessment of mycorrhizae retrieved from sites in the subboreal spruce biogeoclimatic zone in central British Columbia. Mycorrhiza 12:75–82
Acknowledgments
We are grateful to Wang Nian for his helpful working on essay writing, Wu Zhi-Kun for collection in the field, Walter Till and Heimo Rainer (Herbarium of Wien University), as well as Reinhard Agerer and Franck Richard for helpful discussions. We acknowledge David Richardson and two anonymous referees that made helpful and detailed corrections on earlier versions of this paper. This study was supported by the Ministry of Science and Technology of Peoples’ Republic of China (No. 2009GB2F300343) as well as the Chinese plans of New Production of Yunnan (No. 2009BB001) and of Condition Platform Construction of Yunnan (No. 2010 DH011). M.-A. S. is supported by the French Agence Nationale de la Recherche and the CNRS.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Figure S1
The four MH plants investigated in this study. (a) Monotropa uniflora; (b) Hypopitys monotropa; (c) Monotropastrum humile; (d) M. sciaphilum (a, b and d are reproduced from Shen Min et al. 2011). (PDF 181 kb)
Figure S2
Phylogenetic reconstruction of the Strophariaceae family with taxa found in this study (in bold), based on ITS sequences and a Bayesian analysis. Values indicate Bayesian Posterior Probabilities. The tree was rooted by Deconica (Psilocybe) montana as a basal group in Strophariaceae (Bridge et al. 2008). (PDF 163 kb)
Figure S3
Phylogenetic reconstruction of the genus Laccaria with taxa found in this study (in bold), based on ITS sequences and a Bayesian analysis. Values indicate Bayesian Posterior Probabilities. The tree was rooted by Laccaria glabripes, Hydnangium carneum and Porohydnangium australe following the hypothesis of basal origin of Australian lineages of this clade. (PDF 129 kb)
Rights and permissions
About this article
Cite this article
Min, S., Chang-Qin, Z., Yong-Peng, M. et al. Mycorrhizal features and fungal partners of four mycoheterotrophic Monotropoideae (Ericaceae) species from Yunnan, China. Symbiosis 57, 1–13 (2012). https://doi.org/10.1007/s13199-012-0180-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-012-0180-4