Abstract
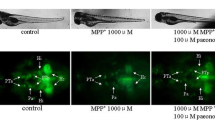
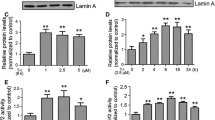
In the present study, necrosis inhibitor-5 (NecroX-5), a novel Cyclopentylamino carboxymethylthiazolylindole (NecroX) series compound was investigated for its protective role against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in a zebrafish model of Parkinson’s disease (PD). MPTP-induced locomotor behavior was measured in zebrafish larvae and the protein expression level of tyrosine hydroxylase (TH) was estimated in zebrafish larva homogenates. MPTP (15 μM) induced a significant (p < 0.05) impairment in zebrafish larvae locomotor behavior. Treatment with NecroX-5 at various doses (3.75, 7.5 and 15 μM) significantly and dose dependently (p < 0.05) restored MPTP-induced locomotor impairments in zebrafish larvae. Further, NecroX-5 significantly attenuated the MPTP-induced decrease in zebrafish TH protein expression levels. The effects observed by NecroX-5 were almost two fold higher when compared with the antioxidant, minocycline. In conclusion, the neuroprotective activity exhibited by NecroX-5 by attenuating MPTP-induced locomotor impairments and dopaminergic TH expression in zebrafish warrants further development of NecroX-5 as a novel neuroprotectant in the treatment of neurodegenerative disorders including PD.




Similar content being viewed by others
References
Anichtchik OV, Kaslin J, Peitsaro N, Scheinin M, Panula P (2004) Neurochemical and behavioural changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurochem 88:443–453
Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3:205–214
Beal MF (2001) Experimental models of Parkinson’s disease. Nat Rev Neurosci 2(5):325–334
Bentivoglio M, Morelli M (2005) The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. In: Dunnett SB, Bentivoglio M, Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy. Elsevier, Amsterdam, pp 1–107
Bové J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2:484–494
Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG (2008) The pathophysiological basis of dystonias. Nat Rev Neurosci 9:222–234
Bretaud S, Lee S, Guo S (2004) Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotoxicol Teratol 26:857–864
Chan P, Di Monte DA, Luo JJ, DeLanney LE, Irwin I, Langston JW (1994) Rapid ATP loss caused by methamphetamine in the mouse striatum: relationship between energy impairment and dopaminergic neurotoxicity. J Neurochem 62:2484–2487
Kim HJ, Koo SY, Ahn B-H et al (2010) NecroX as a novel class of mitochondrial reactive oxygen species and ONOO− scavenger. Arch Pharm Res 33:1813–1823
Li W, Mak M, Jiang H, Wang Q, Pang Y, Chen K, Han Y (2009) Novel anti-Alzheimer’s dimer bis(7)-cognitin: cellular and molecular mechanisms of neuroprotection through multiple targets. Neurother J Am Soc Exp Neurother 6:187–201
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Ma PM (2003) Catecholaminergic systems in the zebrafish. IV. Organization and projection pattern of dopaminergic neurons in the diencephalon. J Comp Neurol 460:13–37
McKinley ET, Baranowski TC, Blavo DO, Cato C, Doan TN, Rubinstein AL (2005) Neuroprotection of MPTP-induced toxicity in zebrafish dopaminergic neurons. Brain Res Mol Brain Res 141:128–137
Nicklas WJ, Vyas I, Heikkila RE (1985) Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci 36:2503–2508
Park MK, Lee BD, Chae SW, Chi J, Kwon SK, Song J-J (2012) Protective effect of NecroX, a novel necroptosis inhibitor, on gentamicin-induced ototoxicity. Int J Pediatr Otorhinolaryngol 76:1265–1269
Parng C, Roy NM, Ton C, Lin Y, McGrath P (2007) Neurotoxicity assessment using zebrafish. J Pharmacol Toxicol Methods 55:103–112
Poli A, Guarnieri T, Facchinetti F, Villani L (1990) Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in goldfish brain. Brain Res 534:45–50
Raslan AA, Kee Y (2013) Tackling neurodegenerative diseases: animal models of Alzheimer’s disease and Parkinson’s disease. Genes Genomics 35:425–440
Rink E, Wullimann MF (2001) The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res 889:316–330
Rink E, Wullimann MF (2002) Development of the catecholaminergic system in the early zebrafish brain: an immunohistochemical study. Brain Res Dev Brain Res 137:89–100
Sallinen V, Torkko V, Sundvik M, Reenilä I, Khrustalyov D, Kaslin J, Panula P (2009) MPTP and MPP + target specific aminergic cell populations in larval zebrafish. J Neurochem 108:719–731
Schapira AHV, Bezard E, Brotchie J et al (2006) Novel pharmacological targets for the treatment of Parkinson’s disease. Nat Rev Drug Discov 5:845–854
Schweitzer J, Lohr H, Filippi A, Driever W (2012) Dopaminergic and noradrenergic circuit development in zebrafish. Dev Neurobiol 72:256–268
Souza BR, Romano-Silva MA, Tropepe V (2011) Dopamine D2 receptor activity modulates Akt signaling and alters GABAergic neuron development and motor behavior in zebrafish larvae. J Neurosci Off J Soc Neurosci 31:5512–5525
Thu VT, Kim H-K, Long LT et al (2012) NecroX-5 prevents hypoxia/reoxygenation injury by inhibiting the mitochondrial calcium uniporter. Cardiovasc Res 94:342–350
Tipton KF, Singer TP (1993) Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J Neurochem 61:1191–1206
Weinreb O, Amit T, Bar-Am O, Youdim MBH (2010) Rasagiline: a novel anti-Parkinsonian monoamine oxidase-B inhibitor with neuroprotective activity. Prog Neurobiol 92:330–344
Winklhofer KF, Haass C (2010) Mitochondrial dysfunction in Parkinson’s disease. Biochim Biophys Acta 1802:29–44
Acknowledgments
The National Research Foundation of Korea (NRF-2010-0012391) supported this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have declared no conflict of interest.
Additional information
Jun-Cheng Liu and Sushruta Koppula contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, JC., Koppula, S., Huh, SJ. et al. Necrosis inhibitor-5 (NecroX-5), attenuates MPTP-induced motor deficits in a zebrafish model of Parkinson’s disease. Genes Genom 37, 1073–1079 (2015). https://doi.org/10.1007/s13258-015-0364-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-015-0364-4