Abstract
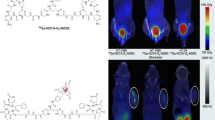
The aim of the study is to evaluate the efficacy of 68Ga-labeled iNGR, containing Asn-Gly-Arg (NGR) homing sequence and CendR (R/KXXR/K) penetrating motif, as a new molecular probe for microPET imaging of CD13-positive xenografts. The synthesized iNGR and NGR peptides were conjugated with DOTA and then labeled with 68Ga. 68Ga-iNGR and 68Ga-NGR were compared in the performance of the in vitro stability, partition coefficient, binding affinity, cell uptake analysis, in vivo microPET imaging, and biodistribution studies in CD13-positive HT-1080 and CD13-negative HT-29 cell lines. The in vitro results revealed that both probes exhibited high radiochemical purity and stability, and no significant difference between two probes was observed in terms of the binding affinity to CD13. In vivo microPET/CT imaging showed that the uptake of 68Ga-iNGR in HT-1080 tumor was significantly higher than that of 68Ga−NGR. Moreover, tumor 68Ga-iNGR uptake could be completely blocked by cold NGR and partially blocked by neutralizing NRP-1 antibody. We concluded that 68Ga-iNGR has a higher tumor uptake and better tumor retention than 68Ga-NGR through NRP-1, indicating that CendR motif modification is a promising method for improving NGR peptide performance.





Similar content being viewed by others
References
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. doi:10.1038/35025220.
Pralhad T, Madhusudan S, Rajendrakumar K. Concept, mechanisms and therapeutics of angiogenesis in cancer and other diseases. J Pharm Pharmacol. 2003;55(8):1045–53. doi:10.1211/0022357021819.
Ellis LM, Liu W, Ahmad SA, Fan F, Jung YD, Shaheen RM, et al. Overview of angiogenesis: biologic implications for antiangiogenic therapy. Semin Oncol. 2001;28(5 Suppl 16):94–104.
Zhang Q, Wang J, Zhang H, Zhao D, Zhang Z, Zhang S. Expression and clinical significance of aminopeptidase N/CD13 in non-small cell lung cancer. J Cancer Res Ther. 2015;11(1):223–8. doi:10.4103/0973-1482.138007.
Wickstrom M, Larsson R, Nygren P, Gullbo J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011;102(3):501–8. doi:10.1111/j.1349-7006.2010.01826.x.
Ikeda N, Nakajima Y, Tokuhara T, Hattori N, Sho M, Kanehiro H, et al. Clinical significance of aminopeptidase N/CD13 expression in human pancreatic carcinoma. Clin Cancer Res. 2003;9(4):1503–8.
Fukasawa K, Fujii H, Saitoh Y, Koizumi K, Aozuka Y, Sekine K, et al. Aminopeptidase N (APN/CD13) is selectively expressed in vascular endothelial cells and plays multiple roles in angiogenesis. Cancer Lett. 2006;243(1):135–43. doi:10.1016/j.canlet.2005.11.051.
Su L, Cao J, Jia Y, Zhang X, Fang H, Xu W. Development of synthetic aminopeptidase N/CD13 inhibitors to overcome cancer metastasis and angiogenesis. ACS Med Chem Lett. 2012;3(12):959–64. doi:10.1021/ml3000758.
Corti A, Curnis F, Arap W, Pasqualini R. The neovasculature homing motif NGR: more than meets the eye. Blood. 2008;112(7):2628–35. doi:10.1182/blood-2008-04-150862.
Chen K, Ma W, Li G, Wang J, Yang W, Yap LP, et al. Synthesis and evaluation of 64Cu-labeled monomeric and dimeric NGR peptides for MicroPET imaging of CD13 receptor expression. Mol Pharm. 2013;10(1):417–27. doi:10.1021/mp3005676.
Ma W, Kang F, Wang Z, Yang W, Li G, Ma X, et al. (99m)Tc-labeled monomeric and dimeric NGR peptides for SPECT imaging of CD13 receptor in tumor-bearing mice. Amino Acids. 2013;44(5):1337–45. doi:10.1007/s00726-013-1469-1.
Shao Y, Liang W, Kang F, Yang W, Ma X, Li G, et al. A direct comparison of tumor angiogenesis with (68)Ga-labeled NGR and RGD peptides in HT-1080 tumor xenografts using microPET imaging. Amino Acids. 2014;46(10):2355–64. doi:10.1007/s00726-014-1788-x.
Zhang J, Lu X, Wan N, Hua Z, Wang Z, Huang H, et al. (68)Ga-DOTA-NGR as a novel molecular probe for APN-positive tumor imaging using MicroPET. Nucl Med Biol. 2014;41(3):268–75. doi:10.1016/j.nucmedbio.2013.12.008.
Mate G, Kertesz I, Enyedi KN, Mezo G, Angyal J, Vasas N, et al. In vivo imaging of Aminopeptidase N (CD13) receptors in experimental renal tumors using the novel radiotracer Ga-NOTA-c(NGR). Eur J Pharm Sci. 2015;69C:61–71. doi:10.1016/j.ejps.2015.01.002.
Werle M, Bernkop-Schnurch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30(4):351–67. doi:10.1007/s00726-005-0289-3.
Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, et al. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16(6):510–20. doi:10.1016/j.ccr.2009.10.013.
Teesalu T, Sugahara KN, Ruoslahti E. Tumor-penetrating peptides. Front Oncol. 2013;3:216. doi:10.3389/fonc.2013.00216.
Feron O. Tumor-penetrating peptides: a shift from magic bullets to magic guns. Sci Transl Med. 2010;2(34):34ps26. doi:10.1126/scitranslmed.3001174.
Alberici L, Roth L, Sugahara KN, Agemy L, Kotamraju VR, Teesalu T, et al. De novo design of a tumor-penetrating peptide. Cancer Res. 2013;73(2):804–12. doi:10.1158/0008-5472.CAN-12-1668.
Ye Y, Zhu L, Ma Y, Niu G, Chen X. Synthesis and evaluation of new iRGD peptide analogs for tumor optical imaging. Bioorg Med Chem Lett. 2011;21(4):1146–50. doi:10.1016/j.bmcl.2010.12.112.
Wester HJ. Nuclear imaging probes: from bench to bedside. Clin Cancer Res. 2007;13(12):3470–81. doi:10.1158/1078-0432.CCR-07-0264.
Liu S, Jia B, Qiao R, Yang Z, Yu Z, Liu Z, et al. A novel type of dual-modality molecular probe for MR and nuclear imaging of tumor: preparation, characterization and in vivo application. Mol Pharm. 2009;6(4):1074–82. doi:10.1021/mp900143a.
Chen X, Liu S, Hou Y, Tohme M, Park R, Bading JR, et al. MicroPET imaging of breast cancer alphav-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol Imaging Biol. 2004;6(5):350–9. doi:10.1016/j.mibio.2004.06.004.
Shi J, Wang L, Kim YS, Zhai S, Liu Z, Chen X, et al. Improving tumor uptake and excretion kinetics of 99mTc-labeled cyclic arginine-glycine-aspartic (RGD) dimers with triglycine linkers. J Med Chem. 2008;51(24):7980–90. doi:10.1021/jm801134k.
Yang Y, Xie X, Yang Y, Zhang H, Mei X. Photo-responsive and NGR-mediated multifunctional nanostructured lipid carrier for tumor-specific therapy. J Pharm Sci. 2015;104(4):1328–39. doi:10.1002/jps.24333.
Wu H, Chen H, Pan D, Ma Y, Liang S, Wan Y, et al. Imaging integrin alphavbeta 3 and NRP-1 positive gliomas with a novel fluorine-18 labeled RGD-ATWLPPR heterodimeric peptide probe. Mol Imaging Biol. 2014;16(6):781–92. doi:10.1007/s11307-014-0761-0.
Menichetti L, Kusmic C, Panetta D, Arosio D, Petroni D, Matteucci M, et al. MicroPET/CT imaging of alphavbeta(3) integrin via a novel (6)(8)Ga-NOTA-RGD peptidomimetic conjugate in rat myocardial infarction. Eur J Nucl Med Mol Imaging. 2013;40(8):1265–74. doi:10.1007/s00259-013-2432-9.
Acevedo LM, Barillas S, Weis SM, Gothert JR, Cheresh DA. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood. 2008;111(5):2674–80. doi:10.1182/blood-2007-08-110205.
Salikhova A, Wang L, Lanahan AA, Liu M, Simons M, Leenders WP, et al. Vascular endothelial growth factor and semaphorin induce neuropilin-1 endocytosis via separate pathways. Circ Res. 2008;103(6):e71–9. doi:10.1161/CIRCRESAHA.108.183327.
Yang Z, Xiang B, Dong D, Wang Z, Li J, Qi X. Dual receptor-specific peptides modified liposomes as VEGF siRNA vector for tumor-targeting therapy. Curr Gene Ther. 2014;14(4):289–99.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant nos. 81230033, 81401442, 81227901, and 81371594), the Post-doctoral Science Foundation of China (grant no. 2015M582802), and the Key Science and Technology Program of Shaanxi Province, China (grant no. 2013K12-03-05). No other potential conflict of interest relevant to this article was reported. We would like to thank Prof. Fan Wang from Medical Isotopes Research Center of Peking University (Beijing, China) for her generous support and Guiyu Li and Changhao Liu for their technical assistance in conducting this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Mingxuan Zhao, Weidong Yang, Mingru Zhang, Fei Kang and Jing Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 877 kb)
Rights and permissions
About this article
Cite this article
Zhao, M., Yang, W., Zhang, M. et al. Evaluation of 68Ga-labeled iNGR peptide with tumor-penetrating motif for microPET imaging of CD13-positive tumor xenografts. Tumor Biol. 37, 12123–12131 (2016). https://doi.org/10.1007/s13277-016-5068-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5068-0