Abstract
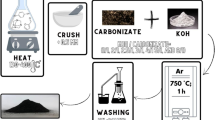
In this research, equilibrium, kinetics, and adsorption mechanism of methylene blue on a relatively large surface area (1437 m2 g−1) Azolla-derived micro- and mesoporous carbon has been investigated. The porous activated carbon was characterized using X-ray diffraction (XRD), Fourier transform infrared (FTIR), Raman and also X-ray photoelectron spectroscopy (XPS), N2 sorption, and high-resolution transmission electron microscopy. Remaining methylene blue solution revealed that the adsorption energy and adsorption capacity of the porous carbon are 21.76 kJ mol−1 and 1930 mg g−1, respectively. Based on the kinetics results, pseudo-second-order kinetic model is dominant during the adsorption process. Diffusion of methylene blue in the porosities and in the space between the graphitic planes of carbons is the rate-limiting parameters. Adsorption of methylene blue into the graphitic planes and the micro-porosities leads to some compressive stresses on the graphitic planes. The relative graphitization value of the prepared carbons will decrease during adsorption process. FTIR and XPS results demonstrate the effect of surface nitrogen groups on the adsorption of methylene blue. During the adsorption process, a relative percent of pyridinic nitrogen will decrease, pyrrolic and graphitic nitrogen of graphitic layers will eliminate and N-(C)3 and H-N-(C)2 nitrogen appear.





Similar content being viewed by others
References
Liu T, Li Y, Du Q, Sun J, Jiao Y, Yang G, Wang Z, Xia Y, Zhang W, Wang K, Zhu H, Wu D (2012) Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf B: Biointerfaces 90:197–203
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Hameed BH, Din ATM, Ahmad AL (2007) Adsorption of methylene blue onto bamboo-based activated carbon: kinetics and equilibrium studies. J Hazard Mater 141:819–825
Hameed BH, Ahmad AL, Latiff KNA (2007) Adsorption of basic dye (methylene blue) onto activated carbon prepared from rattan sawdust. Dyes Pigments 75:143–149
Kornaros M, Lyberatos G (2006) Biological treatment of wastewaters from a dye manufacturing company using a trickling filter. J Hazard Mater 136:95–102
Rashidi HR, Sulaiman NMN, Hashim NA, Hassan CRC, Ramli MR (2015) Synthetic reactive dye wastewater treatment by using nano-membrane filtration. Desalin Water Treat 55:86–95
Djafer A, Djafer L, Maimoun B, Iddou A, Mostefai SK, Ayral A (2017) Reuse of waste activated sludge for textile dyeing wastewater treatment by biosorption: performance optimization and comparison. Water Environ J 31:105–112
Verma AK, Dash RR, Bhunia P (2012) A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J Environ Manag 93:154–168
Sharma P, Hussain N, Borah DJ, Das MR (2013) Kinetics and adsorption behavior of the methyl blue at the graphene oxide/reduced graphene oxide nanosheet–water interface: a comparative study. J Chem Eng Data 58:3477–3488
Han R, Zhang J, Han P, Wang Y, Zhao Z, Tang M (2009) Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem Eng J 145:496–504
Lee YS, Jang W, Koo HY, Choi WS (2015) Facile synthesis of mesoporous SiO2 nanoparticles using the mobility differences of etchants. RSC Adv 5:26223–26230
Mathew A, Parambadath S, Barnabas MJ, Song HJ, Kim J-S, Park SS, Ha C-S (2016) Rhodamine 6G assisted adsorption of metanil yellow over succinamic acid functionalized MCM-41. Dyes Pigments 131:177–185
Lin S, Song Z, Che G, Ren A, Li P, Liu C, Zhang J (2014) Adsorption behavior of metal–organic frameworks for methylene blue from aqueous solution. Microporous Mesoporous Mater 193:27–34
Chen Y, Song X, Zhao T, Xiao Y, Wang Y, Chen X (2018) A phosphorylethanolamine-functionalized super-hydrophilic 3D graphene-based foam filter for water purification. J Hazard Mater 343:298–303
He X, Male KB, Nesterenko PN, Brabazon D, Paull B, Luong JHT (2013) Adsorption and desorption of methylene blue on porous carbon monoliths and Nanocrystalline cellulose. ACS Appl Mater Interfaces 5:8796–8804
Jin Q, Li Y, Yang D, Cui J (2018) Chitosan-derived three-dimensional porous carbon for fast removal of methylene blue from wastewater. RSC Adv 8:1255–1264
Jung K-W, Choi BH, Hwang M-J, Jeong T-U, Ahn K-H (2016) Fabrication of granular activated carbons derived from spent coffee grounds by entrapment in calcium alginate beads for adsorption of acid orange 7 and methylene blue. Bioresour Technol 219:185–195
Tsoncheva T, Mileva A, Tsyntsarski B, Paneva D, Spassova I, Kovacheva D, Velinov N, Karashanova D, Georgieva B, Petrov N (2018) Activated carbon from Bulgarian peach stones as a support of catalysts for methanol decomposition. Biomass Bioenergy 109:135–146
Spessato L, Bedin KC, Cazetta AL, Souza IPAF, Duarte VA, Crespo LHS, Silva MC, Pontes RM, Almeida VC (2019) KOH-super activated carbon from biomass waste: insights into the paracetamol adsorption mechanism and thermal regeneration cycles. J Hazard Mater 371:499–505
Liu J, Liu B, Wang C, Huang Z, Hu L, Ke X, Liu L, Shi Z, Guo Z (2017) Walnut shell–derived activated carbon: synthesis and its application in the sulfur cathode for lithium–sulfur batteries. J Alloys Compd 718:373–378
Liu X, He C, Yu X, Bai Y, Ye L, Wang B, Zhang L (2018) Net-like porous activated carbon materials from shrimp shell by solution-processed carbonization and H3PO4 activation for methylene blue adsorption. Powder Technol 326:181–189
Acosta R, Fierro V, Martinez de Yuso A, Nabarlatz D, Celzard A (2016) Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 149:168–176
Önal Y, Akmil-Başar C, Sarıcı-Özdemir Ç, Erdoğan S (2007) Textural development of sugar beet bagasse activated with ZnCl2. J Hazard Mater 142:138–143
Xu M, Li D, Yan Y, Guo T, Pang H, Xue H (2017) Porous high specific surface area-activated carbon with co-doping N S and P for high-performance supercapacitors. RSC Adv 7:43780–43788
Sayğılı H, Güzel F (2016) High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption. J Clean Prod 113:995–1004
Fan S, Tang J, Wang Y, Li H, Zhang H, Tang J, Wang Z, Li X (2016) Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: kinetics, isotherm, thermodynamic and mechanism. J Mol Liq 220:432–441
Xin W, Song Y, Peng J, Liu R, Han L (2017) Synthesis of biomass-derived Mesoporous carbon with super adsorption performance by an aqueous cooperative assemble route. ACS Sustain Chem Eng 5:2312–2319
Yu R, Shi Y, Yang D, Liu Y, Qu J, Yu Z-Z (2017) Graphene oxide/chitosan aerogel microspheres with honeycomb-cobweb and radially oriented microchannel structures for broad-Spectrum and rapid adsorption of water contaminants. ACS Appl Mater Interfaces 9:21809–21819
Lee JH, Lee HJ, Choi JW (2017) Unveiling anomalous CO2-to-N2 selectivity of graphene oxide. Phys Chem Chem Phys 19:22743–22748
Bedin KC, Martins AC, Cazetta AL, Pezoti O, Almeida VC (2016) KOH-activated carbon prepared from sucrose spherical carbon: adsorption equilibrium, kinetic and thermodynamic studies for methylene blue removal. Chem Eng J 286:476–484
Cazetta AL, Vargas AMM, Nogami EM, Kunita MH, Guilherme MR, Martins AC, Silva TL, Moraes JCG, Almeida VC (2011) NaOH-activated carbon of high surface area produced from coconut shell: kinetics and equilibrium studies from the methylene blue adsorption. Chem Eng J 174:117–125
Liu R-L, Liu Y, Zhou X-Y, Zhang Z-Q, Zhang J, Dang F-Q (2014) Biomass-derived highly porous functional carbon fabricated by using a free-standing template for efficient removal of methylene blue. Bioresour Technol 154:138–147
Pezoti O, Cazetta AL, Bedin KC, Souza LS, Martins AC, Silva TL, Santos Júnior OO, Visentainer JV, Almeida VC (2016) NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: kinetic, isotherm and thermodynamic studies. Chem Eng J 288:778–788
Islam MA, Sabar S, Benhouria A, Khanday WA, Asif M, Hameed BH (2017) Nanoporous activated carbon prepared from karanj (Pongamia pinnata) fruit hulls for methylene blue adsorption. J Taiwan Inst Chem Eng 74:96–104
Li Z, Wang G, Zhai K, He C, Li Q, Guo P (2018) Methylene blue adsorption from aqueous solution by loofah sponge-based porous carbons. Colloids Surf A Physicochem Eng Asp 538:28–35
Marrakchi F, Auta M, Khanday WA, Hameed BH (2017) High-surface-area and nitrogen-rich mesoporous carbon material from fishery waste for effective adsorption of methylene blue. Powder Technol 321:428–434
Elmoubarki R, Mahjoubi FZ, Tounsadi H, Moustadraf J, Abdennouri M, Zouhri A, El Albani A, Barka N (2015) Adsorption of textile dyes on raw and decanted Moroccan clays: kinetics, equilibrium and thermodynamics. Water Resour Ind 9:16–29
Gürses A, Hassani A, Kıranşan M, Açışlı Ö, Karaca S (2014) Removal of methylene blue from aqueous solution using by untreated lignite as potential low-cost adsorbent: kinetic, thermodynamic and equilibrium approach. J Water Process Eng 2:10–21
Islam MA, Ahmed MJ, Khanday WA, Asif M, Hameed BH (2017) Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol Environ Saf 138:279–285
Foo KY, Hameed BH (2012) Preparation, characterization and evaluation of adsorptive properties of orange peel based activated carbon via microwave induced K2CO3 activation. Bioresour Technol 104:679–686
Wang S, Zhai Y-Y, Gao Q, Luo W-J, Xia H, Zhou C-G (2014) Highly efficient removal of acid red 18 from aqueous solution by magnetically retrievable chitosan/carbon nanotube: batch study, isotherms, kinetics, and thermodynamics. J Chem Eng Data 59:39–51
Xu S, Lv Y, Zeng X, Cao D (2017) ZIF-derived nitrogen-doped porous carbons as highly efficient adsorbents for removal of organic compounds from wastewater. Chem Eng J 323:502–511
Maneerung T, Liew J, Dai Y, Kawi S, Chong C, Wang C-H (2016) Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: kinetics, isotherms and thermodynamic studies. Bioresour Technol 200:350–359
Duran C, Ozdes D, Gundogdu A, Senturk HB (2011) Kinetics and isotherm analysis of basic dyes adsorption onto almond Shell (Prunus dulcis) as a low cost adsorbent. J Chem Eng Data 56:2136–2147
Chen L, Bai B (2013) Equilibrium, kinetic, thermodynamic, and in situ regeneration studies about methylene blue adsorption by the raspberry-like TiO2@yeast microspheres. Ind Eng Chem Res 52:15568–15577
Tang H, Yan D, Lu T, Pan L (2017) Sulfur-doped carbon spheres with hierarchical micro/mesopores as anode materials for sodium-ion batteries. Electrochim Acta 241:63–72
Chang P-H, Jiang W-T, Li Z (2018) Mechanism of tyramine adsorption on Ca-montmorillonite. Sci Total Environ 642:198–207
Lv G, Chang P-H, Xing X, Jiang W-T, Jean J-S, Li Z (2017) Investigation of intercalation of diphenhydramine into the interlayer of smectite by XRD FTIR, TG-DTG analyses and molecular simulation. Arab J Chem 10:855–861
Miranda AF, Biswas B, Ramkumar N, Singh R, Kumar J, James A, Roddick F, Lal B, Subudhi S, Bhaskar T, Mouradov A (2016) Aquatic plant Azolla as the universal feedstock for biofuel production. Biotechnol Biofuels 9:221
Perazzolo V, Durante C, Pilot R, Paduano A, Zheng J, Rizzi GA, Martucci A, Granozzi G, Gennaro A (2015) Nitrogen and sulfur doped mesoporous carbon as metal-free electrocatalysts for the in situ production of hydrogen peroxide. Carbon 95:949–963
Tan A-D, Wan K, Wang Y-F, Fu Z-Y, Liang Z-X (2018) N, S-containing MOF-derived dual-doped mesoporous carbon as a highly effective oxygen reduction reaction electrocatalyst. Catal Sci Technol 8:335–343
Li X, Fang Y, Zhao S, Wu J, Li F, Tian M, Long X, Jin J, Ma J (2016) Nitrogen-doped mesoporous carbon nanosheet/carbon nanotube hybrids as metal-free bi-functional electrocatalysts for water oxidation and oxygen reduction. J Mater Chem A 4:13133–13141
Li Y, Wang G, Wei T, Fan Z, Yan P (2016) Nitrogen and sulfur co-doped porous carbon nanosheets derived from willow catkin for supercapacitors. Nano Energy 19:165–175
Kim CK, Choi IT, Kang SH, Kim HK (2017) Anchovy-derived nitrogen and sulfur co-doped porous carbon materials for high-performance supercapacitors and dye-sensitized solar cells. RSC Adv 7:35565–35574
Acknowledgments
We greatly appreciate the help of Rodrigo Fernández-Pacheco from the Universidad de Zaragoza (Spain).
Funding
This research work has been conducted under the kind support of Iran Nanotechnology Initiative Council of Iran University of Science and Technology (IUST).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Motejadded Emrooz, H.B., Maleki, M., Rashidi, A. et al. Adsorption mechanism of a cationic dye on a biomass-derived micro- and mesoporous carbon: structural, kinetic, and equilibrium insight. Biomass Conv. Bioref. 11, 943–954 (2021). https://doi.org/10.1007/s13399-019-00584-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00584-1