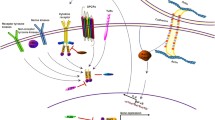
Abstract
StarD13 is a tumor suppressor and a GTPase activating protein (GAP) for Rho GTPases. Thus, StarD13 regulates cell survival pathways and induces apoptosis in a p53-dependent and independent manners. In tumors, StarD13 is either downregulated or completely inhibited, depending on the tumor type. As such, and through the dysregulation of Rho GTPases, this affects adhesion dynamics, actin dynamics, and leads to an increase or a decrease in tumor metastasis depending on the tumor grade and type. Being a key regulatory protein, StarD13 is a potential promising candidate for therapeutic approaches. This paper reviews the key characteristics of this protein and its role in tumor malignancies.


Similar content being viewed by others
References
Ching YP, Wong CM, Chan SF, et al. Deleted in liver cancer (DLC) 2 encodes a RhoGAP protein with growth suppressor function and is underexpressed in hepatocellular carcinoma. J Biol Chem. 2003;278:10824–30.
Lukasik D, Wilczek E, Wasiutynski A, Gornicka B. Deleted in liver cancer protein family in human malignancies (Review). Oncol Lett. 2011;2:763–8.
Durkin ME, Yuan BZ, Zhou X, et al. DLC-1: a Rho GTPase-activating protein and tumour suppressor. J Cell Mol Med. 2007;11:1185–207.
Kwan JJ, Donaldson LW. The NMR structure of the murine DLC2 SAM domain reveals a variant fold that is similar to a four-helix bundle. BMC Struct Biol. 2007;7:34.
Ullmannova V, Popescu NC. Expression profile of the tumor suppressor genes DLC-1 and DLC-2 in solid tumors. Int J Oncol. 2006;29:1127–32.
Bocanegra V, Gil Lorenzo AF, Cacciamani V, Benardon ME, Costantino VV, Valles PG. RhoA and MAPK signal transduction pathways regulate NHE1-dependent proximal tubule cell apoptosis after mechanical stretch. Am J Physiol Renal Physiol. 2014;307:F881–F88989.
Khalil BD, Hanna S, Saykali BA, et al. The regulation of RhoA at focal adhesions by StarD13 is important for astrocytoma cell motility. Exp Cell Res. 2014;321:109–22.
Hanna S, Khalil B, Nasrallah A, et al. StarD13 is a tumor suppressor in breast cancer that regulates cell motility and invasion. Int J Oncol. 2014;44:1499–511.
El-Sitt S, El-Sibai M. The STAR of the DLC family. J Recept Signal Transduct Res. 2013;33:10–3.
El Atat O, Fakih A, El-Sibai M. RHOG activates RAC1 through CDC42 leading to tube formation in vascular endothelial cells. Cells. 2019;8:171.
El-Sitt S, Khalil BD, Hanna S, El-Sabban M, Fakhreddine N, El-Sibai M. DLC2/StarD13 plays a role of a tumor suppressor in astrocytoma. Oncol Rep. 2012;28:511–8.
Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–57.
Srivastava SK, Wheelock RH, Aaronson SA, Eva A. Identification of the protein encoded by the human diffuse B-cell lymphoma (dbl) oncogene. Proc Natl Acad Sci USA. 1986;83:8868–72.
Katzav S, Martin-Zanca D, Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. Embo J. 1989;8:2283–90.
Miki T, Smith CL, Long JE, Eva A, Fleming TP. Oncogene ect2 is related to regulators of small GTP-binding proteins. Nature. 1993;362:462–5.
Whitehead I, Kirk H, Kay R. Expression cloning of oncogenes by retroviral transfer of cDNA libraries. Mol Cell Biol. 1995;15:704–10.
Shebaby WN, Mroueh MA, Boukamp P, et al. Wild carrot pentane-based fractions suppress proliferation of human HaCaT keratinocytes and protect against chemically-induced skin cancer. BMC Complement Altern Med. 2017;17:36.
Singletary K, MacDonald C. Inhibition of benzo[a]pyrene- and 1,6-dinitropyrene-DNA adduct formation in human mammary epithelial cells bydibenzoylmethane and sulforaphane. Cancer Lett. 2000;155:47–544.
Khor TO, Hu R, Shen G, et al. Pharmacogenomics of cancer chemopreventive isothiocyanate compound sulforaphane in the intestinal polyps of ApcMin/+ mice. Biopharm Drug Dispos. 2006;27:407–20.
Zeinab RA, Mroueh M, Diab-Assaf M, et al. Chemopreventive effects of wild carrot oil against 7,12-dimethyl benz(a)anthracene-induced squamous cell carcinoma in mice. Pharm Biol. 2011;49:955–61.
Wolosz D, Walczak A, Szparecki G, et al. Deleted in Liver Cancer 2 (DLC2) protein expression in hepatocellular carcinoma. Eur J Histochem. 2019;63:2981.
Satheesh V, Chidambaranathan P, Jagannadham PT, et al. Transmembrane START domain proteins: in silico identification, characterization and expression analysis under stress conditions in chickpea (Cicer arietinum L.). Plant Signal Behav. 2016;11:e992698.
Sun L, Sun J, Song JD. High expression of DLC family proteins predicts better prognosis and inhibits tumor progression in NSCLC. Mol Med Rep. 2019;19:4881–9.
Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–866.
Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 2000;355:965–70.
Hanna S, El-Sibai M. Signaling networks of Rho GTPases in cell motility. Cell Signal. 2013;25:1955–61.
Li H, Fung KL, Jin DY, et al. Solution structures, dynamics, and lipid-binding of the sterile alpha-motif domain of the deleted in liver cancer 2. Proteins. 2007;67:1154–66.
Zhong D, Zhang J, Yang S, et al. The SAM domain of the RhoGAP DLC1 binds EF1A1 to regulate cell migration. J Cell Sci. 2009;122:414–24.
Cheng C, Feng S, Jiao J, et al. DLC2 inhibits development of glioma through regulating the expression ratio of TAp73alpha/TAp73beta. Am J Cancer Res. 2018;8:1200–13.
Malik-Sheriff RS, Imtiaz S, Grecco HE, Zamir E. Diverse patterns of molecular changes in the mechano-responsiveness of focal adhesions. Sci Rep. 2018;8:2187.
Kawai K, Seike J, Iino T, et al. START-GAP2/DLC2 is localized in focal adhesions via its N-terminal region. Biochem Biophys Res Commun. 2009;380:736–41.
Yang Z, Chen H, Shu M, Zhang Y, Xue L, Lin Y. DLC2 operates as a tumor suppressor gene in breast cancer via the RhoGTPase pathway. Oncol Lett. 2019;17:2107–16.
Salem C, Atallah D, Safi J, et al. Breast density and breast cancer incidence in the Lebanese population: results from a retrospective multicenter study. Biomed Res Int. 2017;2017:7594953.
Nasreddine G, El-Sibai M, Abi-Habib RJ. Cytotoxicity of [HuArgI (co)-PEG5000]-induced arginine deprivation to ovarian cancer cells is autophagy dependent. Investig New Drugs. 2019;38:10–9.
de Tayrac M, Etcheverry A, Aubry M, et al. Integrative genome-wide analysis reveals a robust genomic glioblastoma signature associated with copy number driving changes in gene expression. Genes Chromosom Cancer. 2009;48:55–68.
Wang D, Qian X, Rajaram M, Durkin ME, Lowy DR. DLC1 is the principal biologically-relevant down-regulated DLC family member in several cancers. Oncotarget. 2016;7:45144–57.
Al-Haddad R, Karnib N, Assaad RA, et al. Epigenetic changes in diabetes. Neurosci Lett. 2016;625:64–9.
Sharma A, Kaut O, Pavlova A, et al. Skewed X-chromosome inactivation and XIST locus methylation levels do not contribute to the lower prevalence of Parkinson's disease in females. Neurobiol Aging. 2017;57:248.e1–48.e5.
Shammas H, Kuech E-M, Rizk S, Das AM, Naim HY. Different Niemann-Pick C1 genotypes generate protein phenotypes that vary in their intracellular processing, trafficking and localization. Sci Rep. 2019;9:5292.
Hankins GR, Sasaki T, Lieu AS, et al. Identification of the deleted in liver cancer 1 gene, DLC1, as a candidate meningioma tumor suppressor. Neurosurgery. 2008;63:771–80 (discussion 80-1).
Harden SV, Tokumaru Y, Westra WH, et al. Gene promoter hypermethylation in tumors and lymph nodes of stage I lung cancer patients. Clin Cancer Res. 2003;9:1370–5.
Peng H, Long F, Wu Z, et al. Downregulation of DLC-1 gene by promoter methylation during primary colorectal cancer progression. Biomed Res Int. 2013;2013:181384.
El-Sibai M, Pertz O, Pang H, et al. RhoA/ROCK-mediated switching between Cdc42- and Rac1-dependent protrusion in MTLn3 carcinoma cells. Exp Cell Res. 2008;314:1540–52.
Fagan-Solis KD, Schneider SS, Pentecost BT, et al. The RhoA pathway mediates MMP-2 and MMP-9-independent invasive behavior in a triple-negative breast cancer cell line. J Cell Biochem. 2013;114:1385–94.
Li W, Chong H, Guan KL. Function of the Rho family GTPases in Ras-stimulated Raf activation. J Biol Chem. 2001;276:34728–37.
Morrison DK, Cutler RE. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–9.
Leung TH, Yam JW, Chan LK, Ching YP, Ng IO. Deleted in liver cancer 2 suppresses cell growth via the regulation of the Raf-1-ERK1/2-p70S6K signalling pathway. Liver Int. 2010;30:1315–23.
Zhang H, Wang F, Hu Y. STARD13 promotes hepatocellular carcinoma apoptosis by acting as a ceRNA for Fas. Biotechnol Lett. 2017;39:207–17.
Pezeshkpoor B, Berkemeier AC, Czogalla KJ, Oldenburg J, El-Maarri O. Evidence of pathogenicity of a mutation in 3′ untranslated region causing mild haemophilia A. Haemophilia. 2016;22:598–603.
Guo X, Xiang C, Zhang Z, Zhang F, Xi T, Zheng L. Displacement of Bax by BMF Mediates STARD13 3′UTR-Induced Breast Cancer Cells Apoptosis in an miRNA-Depedent Manner. Mol Pharm. 2018;15:63–71.
Laufs U, Marra D, Node K, Liao JK. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1). J Biol Chem. 1999;274:21926–31.
Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–30.
Seasholtz TM, Zhang T, Morissette MR, Howes AL, Yang AH, Brown JH. Increased expression and activity of RhoA are associated with increased DNA synthesis and reduced p27(Kip1) expression in the vasculature of hypertensive rats. Circ Res. 2001;89:488–95.
Muppani N, Nyman U, Joseph B. TAp73alpha protects small cell lung carcinoma cells from caspase-2 induced mitochondrial mediated apoptotic cell death. Oncotarget. 2011;2:1145–54.
Vikhreva P, Melino G, Amelio I. p73 alternative splicing: exploring a biological role for the C-terminal isoforms. J Mol Biol. 2018;430:1829–38.
El-Amm J, Aragon-Ching JB. Targeting bone metastases in metastatic castration-resistant prostate cancer. Clin Med Insights Oncol. 2016;10:11–9.
Daaboul HE, Dagher C, Taleb RI, et al. The chemotherapeutic effect of beta-2-himachalen-6-ol in chemically induced skin tumorigenesis. Biomed Pharmacother. 2018;103:443–52.
Nader R, El Amm J, Aragon-Ching JB. Role of chemotherapy in prostate cancer. Asian J Androl. 2018;20:221–9.
Audi H, Azar DF, Mahjoub F, et al. Cytotoxicity modulation of ruthenium(II) tris-bathophenanthroline complexes with systematically varied charge. J Photochem Photobiol A. 2018;351:59–68.
Abou-Antoun TJ, Hale JS, Lathia JD, Dombrowski SM. Brain cancer stem cells in adults and children: cell biology and therapeutic implications. Neurotherapeutics. 2017;14:372–84.
Abou-Antoun TJ, Nazarian J, Ghanem A, Vukmanovic S, Sandler AD. Molecular and functional analysis of anchorage independent, treatment-evasive neuroblastoma tumorspheres with enhanced malignant properties: a possible explanation for radio-therapy resistance. PLoS One. 2018;13:e0189711.
Khalife R, Hodroj MH, Fakhoury R, Rizk S. Thymoquinone from Nigella sativa seeds promotes the antitumor activity of noncytotoxic doses of topotecan in human colorectal cancer cells in vitro. Planta Med. 2016;82:312–21.
Najar M, Fayyad-Kazan H, Faour WH, et al. Human hepatic stellate cells and inflammation: a regulated cytokine network balance. Cytokine. 2017;90:130–4.
Al-Dimassi S, Salloum G, Saykali B, et al. Targeting the MAP kinase pathway in astrocytoma cells using a recombinant anthrax lethal toxin as a way to inhibit cell motility and invasion. Int J Oncol. 2016;48:1913–20.
Braun AC, Olayioye MA. Rho regulation: DLC proteins in space and time. Cell Signal. 2015;27:1643–51.
Vitiello E, Ferreira JG, Maiato H, Balda MS, Matter K. The tumour suppressor DLC2 ensures mitotic fidelity by coordinating spindle positioning and cell-cell adhesion. Nat Commun. 2014;5:5826.
Faour WH, Fayyad-Kazan H, El Zein N. fMLP-dependent activation of Akt and ERK1/2 through ROS/Rho A pathways is mediated through restricted activation of the FPRL1 (FPR2) receptor. Inflamm Res. 2018;67:711–22.
Al Hassan M, Fakhoury I, El Masri Z, et al. Metformin treatment inhibits motility and invasion of glioblastoma cancer cells. Anal Cell Pathol (Amst). 2018;2018:5917470.
Jin X, Zhu Z, Shi Y. Metastasis mechanism and gene/protein expression in gastric cancer with distant organs metastasis. Bull Cancer. 2014;101:E1–.
Fayyad-Kazan H, Faour WH, Badran B, Lagneaux L, Najar M. The immunomodulatory properties of human bone marrow-derived mesenchymal stromal cells are defined according to multiple immunobiological criteria. Inflamm Res. 2016;65:501–10.
Zaatiti H, Abdallah J, Nasr Z, Khazen G, Sandler A, Abou-Antoun TJ. Tumorigenic proteins upregulated in the MYCN-amplified IMR-32 human neuroblastoma cells promote proliferation and migration. Int J Oncol. 2018;52:787–803.
Takaoka M, Ito S, Miki Y, Nakanishi A. FKBP51 regulates cell motility and invasion via RhoA signaling. Cancer Sci. 2017;108:380–9.
Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–60.
Basak P, Leslie H, Dillon RL, Muller WJ, Raouf A, Mowat MRA. In vivo evidence supporting a metastasis suppressor role for Stard13 (Dlc2) in ErbB2 (Neu) oncogene induced mouse mammary tumors. Genes Chromosomes Cancer. 2018;57:182–91.
Prudnikova TY, Rawat SJ, Chernoff J. Molecular pathways: targeting the kinase effectors of RHO-family GTPases. Clin Cancer Res. 2015;21:24–9.
Zhou FQ, Snider WD. Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci. 2006;361:1575–92.
El-Sibai M, Nalbant P, Pang H, et al. Cdc42 is required for EGF-stimulated protrusion and motility in MTLn3 carcinoma cells. J Cell Sci. 2007;120:3465–74.
Fostok SF, El-Sibai M, El-Sabban M, Talhouk RS. Gap junctions and Wnt signaling in the mammary gland: a cross-talk? J Mammary Gland Biol Neoplasia. 2019;24:17–38.
Stengel K, Zheng Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal. 2011;23:1415–23.
Yamaguchi H, Pixley F, Condeelis J. Invadopodia and podosomes in tumor invasion. Eur J Cell Biol. 2006;85:213–8.
Lin Y, Chen NT, Shih YP, Liao YC, Xue L, Lo SH. DLC2 modulates angiogenic responses in vascular endothelial cells by regulating cell attachment and migration. Oncogene. 2010;29:3010–6.
Tang F, Zhang R, He Y, Zou M, Guo L, Xi T. MicroRNA-125b induces metastasis by targeting STARD13 in MCF-7 and MDA-MB-231 breast cancer cells. PLoS ONE. 2012;7:e35435.
Chang S, He S, Qiu G, et al. MicroRNA-125b promotes invasion and metastasis of gastric cancer by targeting STARD13 and NEU1. Tumour Biol. 2016;37:12141–51.
Li X, Zheng L, Zhang F, et al. STARD13-correlated ceRNA network inhibits EMT and metastasis of breast cancer. Oncotarget. 2016;7:23197–211.
Najar M, Fayyad-Kazan H, Faour WH, Badran B, Journe F, Lagneaux L. Breast cancer cells and bone marrow mesenchymal stromal cells: a regulated modulation of the breast tumor in the context of immune response. Inflamm Res. 2017;66:129–39.
Najar M, Fayyad-Kazan H, Faour WH, et al. Immunological modulation following bone marrow-derived mesenchymal stromal cells and Th17 lymphocyte co-cultures. Inflamm Res. 2019;68:203–13.
Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. Faseb J. 1999;13:9–22.
Sellami N, Lamine LB, Turki A, et al. Association of VEGFA variants with altered VEGF secretion and type 2 diabetes: a case–control study. Cytokine. 2018;106:29–34.
Hu J, Li X, Guo X, et al. The CCR2 3'UTR functions as a competing endogenous RNA to inhibit breast cancer metastasis. J Cell Sci. 2017;130:3399–413.
Yuan BZ, Miller MJ, Keck CL, Zimonjic DB, Thorgeirsson SS, Popescu NC. Cloning, characterization, and chromosomal localization of a gene frequently deleted in human liver cancer (DLC-1) homologous to rat RhoGAP. Cancer Res. 1998;58:2196–9.
Kim TY, Vigil D, Der CJ, Juliano RL. Role of DLC-1, a tumor suppressor protein with RhoGAP activity, in regulation of the cytoskeleton and cell motility. Cancer Metastasis Rev. 2009;28:77–83.
Cao X, Voss C, Zhao B, Kaneko T, Li SS. Differential regulation of the activity of deleted in liver cancer 1 (DLC1) by tensins controls cell migration and transformation. Proc Natl Acad Sci USA. 2012;109:1455–60.
Acknowledgements
The authors thank the Lebanese American University. The authors were supported by the Lebanese American University, Department of Natural science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jaafar, L., Chamseddine, Z. & El-Sibai, M. StarD13: a potential star target for tumor therapeutics. Human Cell 33, 437–443 (2020). https://doi.org/10.1007/s13577-020-00358-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00358-2