Abstract
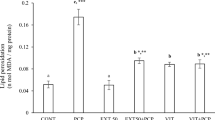
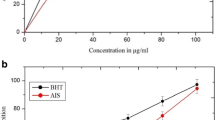
Free radical oxygen species cause protein carbonylation, an irreversible oxidative damage associated with diseases such as liver injury, diabetes mellitus, arthritis, cancer and Alzheimer. To determine the inhibitory activity of the protein carbonylation and the hepatoprotective effect of ethanol extract of the fruits of Caesalpinia coriaria Jacq (Fabaceae). Scavenging activity of 2,2-diphenyl-1- picrylhydrazyl radical (DPPH•) and the radical cation 2,2’-azino-bis(3-ethylbenzothiazoline-sulfonic acid) (ABTS•+) of the extract of C. coriaria at 500 mg / L, was evaluated in a preliminary test. Inhibitory activity of the protein carbonylation isolated from mouse liver using Western methodology with dinitrophenylhydrazine (DNPH) probes was evaluated. The hepatoprotective activity of the extract was evaluated in vivo in Wistar rats using carbon tetrachloride (CCl4) as toxic orally. The degree of hepatoprotection was determined by measuring the serum liver enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and by histopathological analysis of rat liver. The rate of capture of the radicals DPPH• and ABTS•+ by the extract of C. coriaria was higher than 90 % in both cases. The extract also showed a better inhibitory effect on the carbonylation of the proteins than butyl hydroxytoluene (BHT) used as positive control. The extract of C. coriaria produced a significant reduction of AST concentration (from 1667,67 ± 394,27 to 718,00 ± 24,85 U/L ) and ALT (from 967,67 ± 118,30 to 625.67 ± 60,98 U/L), indicating liver cell damage attenuation. The extract of C. coriaria produced an important antioxidant activity and moderate hepatoprotective effect against CCl4-induced toxicity.


Similar content being viewed by others
References
Adisakwattana S, Thilavech T, Chusak Ch (2014) Mesona Chinensis Benth extract prevents AGE formation and protein oxidation against fructose-induced protein glycation in vitro. BMC Complementary and Alternative Medicine [Online]. Available at:http://www.biomedcentral.com/1472-6882/14/130Accessed. 12 May 2015
Anandhi D, Revathi K (2013) Phytochemical analysis of Caesalpinia coriaria (Jacq) wild. Int J Biosci Res 2(1):1–7
Arts IC, Hollman PC, Feskens EJ (2011) Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen elderly study. Am J Clin Nutr 74:227–232
Bachi A, Dalle-Done I, Scaloni A (2013) Redox proteomics: chemical principles, methodological approaches and biological/biomedical promises. Chem Rev 113:596–698
Berlett BS, Stadtman ER (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272(33):20313–20316
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chang YC, Chuang LM (2010) The role of oxidative stress in the pathogenesis of type 2 diabetes: from molecular mechanism to clinical implication. Am J Transl Res 2(3):316–331
Codreanu SG, Liebler DC (2015) Novel approaches to identify protein adducts produced by lipid peroxidation. Free Radic Res 1–7
Curtis JM, Hahn WS, Long EK et al (2012) Protein carbonylation and metabolic control systems. Trends Endocrinol Metab 23(8):399–406
Dalle-Donne I, Giustarini D, Colombo R et al (2003) Protein carbonylation in human diseases. Trends Mol Med 9(4):169–176
Dewanto V, Wu X, Adom KK et al (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014
Di Domenico F, Coccia R, Butterfield DA et al (2011) Circulating biomarkers of protein oxidation for Alzheimer disease: expectations within limits. Biochim Biophys Acta 1814:1785–1795
Estévez M (2011) Protein carbonyls in meat systems: a review. Meat Sci 89:259–279
Freeman BA, Crapo JD (1982) Biology of disease: free radicals and tissue injury. Lab Investig 47:412–426
Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344(8924):721–724
Hense HW, Stendera M, Borsc W et al (1993) Lack of an association between serum vitamin E and myocardial infarction in a population with high vitamin E levels. Atherosclerosis 103(1):21–28
Hertog MGL, Feskens EJM, Hollman PCH et al (1993) Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen elderly study. Lancet 342:1007–1011
Hopps E, Noto D, Caimi G et al (2010) A novel component of the metabolic syndrome: the oxidative stress. Nutr Metab Cardiovasc Dis 20:72–77
Lee C-P, Shih P-H, Hsu C-L et al (2007) Hepatoprotection of tea seed oil (Camellia oleifera Abel.) against CCl4-induced oxidative damage in rats. Food Chem Toxicol 45:888–895
Lokeswari N, Sujatha P (2011) Isolation of tannins from Caesalpinia coriaria and effect of physical parameters. Int Res J Pharm 2(2):146–152
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its 5 classification. Chem Biol Interact. doi:10.1016/j.cbi.2014.10.016, Article in press
Madhu Kiran P, Vijaya Raju A, Ganga Rao B (2012). Investigation of hepatoprotective activity of Cyathea gigantea (Wall. ex. Hook.) leaves against paracetamol-induced hepatotoxicity in rats. Asian Pac J Trop Biomed 352–356
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 23:134–147
Mendez D, Linares M, Diez A et al (2011) Stress response and cytoskeletal proteins involved in erythrocyte membrane remodelling upon Plasmodium falciparum invasion are differentially carbonylated in G6PD A deficiency. Free Radic Biol Med 50(10):1305–1313
Møller IM, Rogowska-Wrzesinska A, Rao RSP (2011) Protein carbonylation and metal-catalyzed protein oxidation in a cellular perspective. J Proteomics 74:2228–2242
Navarro VJ, Senior JR (2006) Drug-related hepatotoxicity. N Engl J Med 354(7):731–739
Niki E (2009) Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med 47:469–484
Orhan IE, Sener B, Musharraf SG (2012) Antioxidant and hepatoprotective activity appraisal of four selected Fumaria species and their total phenol and flavonoid quantities. Exp Toxicol Pathol 64:205–209
Ravikumar S, Gnanadesigan M (2012) Hepatoprotective and antioxidant properties of rhizophora mucronata mangrove plant in CCl4 intoxicated rats. J Exp Clin Med 4(1):66–72
Re R, Pellegrini N, Proteggente A et al (1999) Antioxidant activity applying an improved ABTS radical cation decollorization assay. Free Radic Biol Med 26:1231–1237
Seki S, Kitada T, Sakaguhi H et al (2003) Pathological significance of oxidative cellular damage in human alcoholic liver disease. Histopathology 42:365–371
Shaker E, Mahmoud H, Mnaa S (2010) Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol 48:803–806
Shukla S, Mehta A, John J et al (2009) Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem Toxicol 47:1848–1851
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295
Silva B, Andrade P, Valentao P et al (2004) Quince (Cydonia oblonga Miller) fruit (pulp, peel, and seed) and jam: antioxidant activity. J Agric Food Chem 52:4705–4712
Sivasankari K, Janaky S, Sekar T (2011) Antioxidant status of leaves of Caesalpinia bonduc. Int J Pharm Appl 2(4):262–266
Vargas F, Rivas C, Nursama A et al (2007) Reacciones de radicales libres con relevancia biológica en la teoría del envejecimiento. Av Quím 2(2):3–15, Universidad de los Andes Mérida Venezuela
Voulgaridoua G-P, Anestopoulosa I, Francob R et al (2011) DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat Res 711:13–27
Yochum L, Kushi LH, Meyer K et al (1999) Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol 149:943–949
Acknowledgments
The authors thank to the University of Cartagena for funding this study; we also thank to the University Hospital of the Caribbean and to the Clinical Laboratory Eduardo Fernandez, for technical and scientific support. We also thank Mr. Moises Carrascal Medina and Miss Adriana Tinoco for collecting the plant material used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All experiments were performed at the University of Cartagena and the Laboratory of Pathology of the University Hospital of Caribbean, according to the guidelines of the International Council for Laboratory Animal and were approved by the ethical committee of the University Hospital of Caribbean, Cartagena, Colombia (Act No. 04, approval date 14 may 2012).
Conflict of Interests
The authors declare no conflict of Interest.
Rights and permissions
About this article
Cite this article
Pájaro González, Y., Méndez Cuadro, D., Fernández Daza, E. et al. Inhibitory activity of the protein carbonylation and hepatoprotective effect of the ethanol-soluble extract of Caesalpinia coriaria Jacq. Orient Pharm Exp Med 16, 225–232 (2016). https://doi.org/10.1007/s13596-016-0228-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-016-0228-8