Abstract
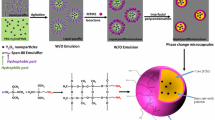
The encapsulation of phase change materials (PCMs) as thermal energy storage materials is a big issue. PCM is usually encapsulated to avoid spillage, flammability and its reaction with the surrounding environment to improve its application. In the last decade, various methods have been employed and all kinds of microencapsulated PCM are produced. In this paper, we present a facile route to produce an encapsulated PCM with an organic and inorganic shell. The encapsulated phase change material (PCM) was prepared using a coaxial micro-fluidic system combined with an ionic cross-linking process. The alginate was used as the basic shell and a range of capsules was obtained by modifying the original shell using two inorganic components such as sodium carbonate and sodium silicate. Various samples, each with a different surrounding layer, were prepared by combining alginate calcium (Alg–Ca) as an organic shell with an inorganic component such as alginate calcium carbonate (Alg–CaCO3) and alginate calcium silicate (Alg–CaSiO3). In these experimental works, we have investigated the compatibility and the stability of capsules modified with the inorganic component. The scanning electron microscopy (SEM) technique and optical microscopy were utilized to study the capsule morphology. The chemical composition of the shell was evaluated by Fourier transform infrared spectroscopy (ATR-FTIR), thermogravimetry analysis and SEM coupled with the EDX analysis, and the capsule stability was estimated under an accelerated thermal cycling.











Similar content being viewed by others
Change history
18 May 2020
The article listed above was initially published with typo error in first author name.
References
Alva G, Lin Y, Liu L, Fang G (2017) Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage. Energ Build 144:276–294
Srinivasaraonaik B, Singh LP, Tyagi I, Anujay R, Shishir S (2020) Microenca psulation of a eutectic PCM using in situ polymerization technique for thermal energy storage. Int J Energy Res 20:1–11
Li X, Li Y (2015) Applications of organic phase change materials embedded in adsorbents for controlling heat produced by charging and discharging natural gas. Adsorption 21:383–389
Qian T, Dang B, ChenY JC, Qian J, Sun Q (2019) Fabrication of magnetic phase change n-eicosane @ Fe3O4/SiO2 microcapsules on wood surface via sol-gel method. J Alloys Compd 772:871–876
Guo X, Cao J, Peng Y, Liu R (2016) Incorporation of microencapsulated dodecanol into wood flour/high-density polyethylene composite as a phase change material for thermal energy storage. Mater Des 89:1325–1334
Waterhouse GI, Wang W, Sun-Waterhouse D (2014) Stability of canola oil encapsulated by co-extrusion technology: effect of quercetin addition to alginate shell or oil core. Food Chem 142:27–38
Moghaddam MK, Mortazavi SM, Khaymian T (2015) Micro/nano-encapsulation of a phase change material by coaxial electrospray method. Iran Polym 24:759–774
Liu H, Wang X, Wu D, Ji S (2019) Fabrication and applications of dual-responsive microencapsulated phase change material with enhanced solar energy-storage and solar photocatalytic effectiveness. Sol Energ Mat Sol C 193:184–197
Zhao L, Luo J, Wang H, Song G, Tang G (2016) Self-assembly fabrication of microencapsulated n-octadecane with natural silk fibroin shell for thermal-regulating textiles. Appl Therm Eng 99:495–501
Moon SK, Cheong IW, Choi SW (2014) Effect of flow rates of the continuous phase on droplet size in dripping and jetting regimes in a simple fluidic device for coaxial flow. Colloids Surf A Physicochem Eng Asp 454:84–88
Mignon A, De Belie N, Dubruel P, Van Vlierberghe S (2019) Superabsorbent polymers: a review on the characteristics and applications of synthetic, polysaccharide-based, semi-synthetic and “smart” derivatives. Eur Polym J 117:165–178
Liu S, Li L (2017) Effect of functionalized graphene oxide on gelation and scaling law of alginate in aqueous solution. Eur Polym J 95:462–473
Goh CH, Heng PWS, Chan LW (2012) Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr Polym 88:1–12
Leong JY, Lam WH, Ho KW, Voo WP, Lee MFX, Lim HP, Lim SL, Tey BT, Poncelet D, Chan ES (2016) Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology 24:44–60
Bienaimé C, Barbotin JN, Nava-Saucedo JE (2003) How to build an adapted and bioactive cell microenvironment? A chemical interaction study of the structure of Ca-alginate matrices and their repercussion on confined cells. J Biomed Mater Res A 67A:376–388
Birdi G, Bridson RH, Smith AM, Bohari SPM, Grover LM (2012) Modification of alginate degradation properties using orthosilicic acid. J Mech Behav Biomed 6:181–187
Angelozzi M, Miotto M, Penolazzi L, Mazzitelli S, Keane T, Badylak SF, Piva R, Nastruzzi C (2015) Composite ECM–alginate microfibers produced by microfluidics as scaffolds with biomineralization potential. Mater Sci Eng C 56:141–153
Ramdhan T, Ching SH, Prakash S, Bhandari B (2019) Time dependent gelling properties of cuboid alginate gels made by external gelation method: effects of alginate-CaCl2 solution ratios and pH. Food Hydrocoll 90:232–240
Abdelaal HM (2014) Fabrication of hollow silica microspheres utilizing a hydrothermal approach. Chin Chem Lett 25:627–629
Zhang H, Sun S, Wang X, Wu D (2011) Fabrication of microencapsulated phase change materials based on n-octadecane core and silica shell through interfacial polycondensation. Colloids Surf A Physicochem Eng Asp 389:104–117
He F, Wang X, Wu D (2014) New approach for sol–gel synthesis of microencapsulated n-octadecane phase change material with silica wall using sodium silicate precursor. Energy 67:223–233
Shim J, Lim JM, Shea PJ, Oh BT (2014) Simultaneous removal of phenol, Cu and Cd from water with corn cob silica-alginate beads. J Hazard Mater 272:129–136
Benedini L, Placente D, Pieroni O, Messina P (2017) Assessment of synergistic interactions on self-assembled sodium alginate/nano-hydroxyapatite composites: to the conception of new bone tissue dressings. Colloid Polym Sci 295:2109–2121
Bhagavatheswaran ES, Das A, Rastin H, Saeidi H, Jafari SH, Vahabi H, Najafi F, Khonakdar HA, Formela K, Jouyandeh M, Zarrintaj P, Saeb MR (2019) The taste of waste: the edge of eggshell over calcium carbonate in acrylonitrile butadiene rubber. J Polym Environ 27:2478–2489
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised to correct the first author name.
Rights and permissions
About this article
Cite this article
Miloudi, R., Zerrouki, D. Encapsulation of phase change materials with alginate modified by nanostructured sodium carbonate and silicate. Iran Polym J 29, 543–550 (2020). https://doi.org/10.1007/s13726-020-00819-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-020-00819-3