Abstract
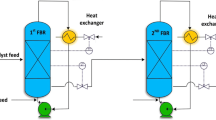
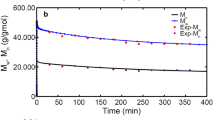
A hierarchical and computationally efficient mathematical model was developed to explain the polymerization of high-density polyethylene (HDPE) in an isothermal, industrial, continuous stirred tank slurry reactor (CSTR). A modified polymeric multi-grain model (PMGM) was used. Steady-state macroscopic mass balance equations were derived for all species (namely, monomer, solvent, catalyst and polymer) to obtain the final particle size and the required monomer and solvent input rates for a given catalyst input and the reactor residence time. The interphase mass transfer coefficients were calculated for the industrial CSTR using the operating data on the reactor. The present model was tuned with some data on an isothermal industrial reactor and the simulation results were compared with data on another set of industrial reactor. The comparison revealed that the present tuned model is capable of predicting the productivity and the polymer yield at various catalyst feed rates and the mean residence times. The effects of variation of two operating variables (catalyst feed rate and mean residence time) on the productivity, the polymer yield, the polydispersity index (PDI) and the operational safety were analyzed. The present study indicated that an optimal value of the reactor residence time (for maximum productivity per catalyst particle) exists at any catalyst feed rate.








Similar content being viewed by others
Abbreviations
- a gl :
-
Interfacial area of gas/liquid interface (m2/m3)
- a ls :
-
Interfacial area of liquid/solid interface (m2/m3)
- C * :
-
Concentration of catalyst active site (kmol site/m3 of catalyst)
- d a :
-
Diameter of the impeller (m)
- d b :
-
Average diameter of gas bubbles (m)
- D 1 :
-
Diffusivity of monomer in pure polymer (m2/s)
- D cat :
-
Diameter of original catalyst particle (m)
- D ef :
-
Effective diffusivity of monomer inside the macroparticle (m2/s)
- D ef,i :
-
Effective diffusivity of monomer inside the macroparticle at the ith grid point (m2/s)
- D L :
-
Diffusivity of monomer in the bulk liquid (m2/s)
- D mp :
-
Diameter of macroparticle (m)
- D n :
-
Concentration of dead polymer chains of n monomeric units (kmol/m3 catalyst)
- \(D_{{{\text{mp}}}}^{{{\text{plant}}}}\) :
-
Diameter of macroparticle from industrial reactor data (m)
- \(D_{{{\text{mp}}}}^{{{\text{ref}}}}\) :
-
Reference value of the diameter of macroparticle (m)
- D R :
-
Diameter of the reactor (m)
- F :
-
Volumetric fraction of solids in the slurry (m3 of solid/m3 of slurry)
- f c :
-
Mass fraction of catalyst present in the solid (kg cat/kg solid)
- H2 :
-
Hydrogen concentration (kmol/m3)
- \({\text{H}}_{{\text{et-hex}}}\) :
-
Henry’s law constant of ethylene in n-hexane (Pa m3/kmol)
- \({\text{H}}_{{{\text{H}}_{{2}} {\text{-hex}}}}\) :
-
Henry’s law constant of hydrogen in n-hexane (Pa m3/kmol)
- \(I_{{\text{C,in}}}\) :
-
Rate of catalyst input (kg/s)
- \(I_{{\text{M,in}}}\) :
-
Rate of monomer input (kg/s)
- \(I_{{\text{M,in}}}^{{{\text{ref}}}}\) :
-
Reference value of the rate of monomer input from industrial reactor data (kg/s)
- \(I_{{\text{S,in}}}\) :
-
Rate of the solvent input (kg/s)
- \(I_{{\text{S,in}}}^{{{\text{ref}}}}\) :
-
Reference value of the rate of solvent input from industrial reactor data (kg/s)
- k gl :
-
Mass transfer coefficient at gas/liquid interface (m/s)
- \(k_{{{\text{ls}}}}\) :
-
Mass transfer coefficient at liquid/solid interface (m/s)
- k P :
-
Rate constant for propagation (m3 kmol−1 s−1)
- k tr :
-
Rate constant for chain transfer to H2 (m1.5 kmol−0.5 s−1)
- M * :
-
Equilibrium monomer concentration (kmol/m3)
- M L :
-
Molar concentration of monomer in the bulk of the reacting liquid (kmol/m3)
- \(M_{{\text{n}}}\) :
-
Number average molecular weight (kg/kmol)
- M P + 2 :
-
Monomer concentration at the outer surface of the macroparticle (kmol/m3)
- \(\overline{M}_{n}\) :
-
Mean value of the number average molecular weight (kg/kmol)
- \(M_{{\text{w}}}\) :
-
Weight average molecular weight (kg/kmol)
- \(\overline{M}_{w}\) :
-
Mean value of the weight average molecular weight (kg/kmol)
- MW:
-
Molecular weight of the monomer (kg/kmol)
- N E :
-
Total number of catalyst particles entering the reactor per second (s−1)
- N F :
-
Dimensionless flow number
- N P :
-
Dimensionless power number
- N S :
-
Speed of impeller (rps)
- N i :
-
Number of sub-particles in the ith shell
- P :
-
Number of computational shells
- P 0 :
-
Concentration of empty sites (kmol/m3 catalyst)
- \(P_{{\text{d}}}\) :
-
Power delivered to liquid (W)
- p et :
-
Partial pressure of ethylene in the vapor phase (Pa)
- p H 2 :
-
Partial pressure of hydrogen in the vapor phase (Pa)
- p hex :
-
Partial pressure of n-hexane in the vapor phase (Pa)
- \(P_{{\text{S}}}\) :
-
Power delivered to the impeller shaft (W)
- P t :
-
Total pressure inside the reactor (Pa)
- PDI:
-
Polydispersity index
- \(\overline{\text{PDI}}\) :
-
Mean value of polydispersity index
- Q T :
-
Rate of withdrawal of product (slurry) (m3/s)
- R c,av :
-
Average radius of catalyst sub-particles (m)
- R c, i :
-
Radius of catalyst sub-particle in the ith shell (m)
- Re:
-
Reynolds number
- R gl :
-
Rate of monomer transfer from the gas phase to the liquid phase (kmol/s)
- R ls :
-
Rate of monomer transfer from the liquid phase to the solid phase (kmol/s)
- R h, i :
-
Radius of macroparticle at the hypothetical grid point i (m)
- R c,max :
-
Maximum radius of catalyst sub-particles (m)
- R mp :
-
Polymer production rate of each catalyst particle (kmol/cat particle s)
- R poly :
-
Rate of formation of polymer inside the reactor (kg/s)
- R v :
-
Rate of reaction per unit volume (kmol/m3 s)
- R v ,i :
-
Rate of reaction per unit volume at the ith grid point (kmol/m3 s)
- Sc:
-
Schmidt number
- Sh:
-
Sherwood number
- u s :
-
Velocity of gas bubble (m/s)
- \(v_{{\text{C}}}\) :
-
Volume of one catalyst particle (m3)
- \(v_{{\text{G}}}\) :
-
Total volume of gas dissolved in the n-hexane (m3)
- \(v_{{\text{L}}}\) :
-
Total volume of liquid in the slurry (m3)
- \(v_{{\text{s}}}\) :
-
Total volume of solid in the slurry (m3)
- V R :
-
Volume of the reactor (m3)
- \({\upalpha }\) :
-
Probability of propagation
- \(\theta\) :
-
Mean residence time of the reactor (s)
- \(\lambda\) :
-
Moment of the live polymer chains (kg/kmol)
- \(\mu\) :
-
Moment of the dead polymer chains (kg/kmol)
- \(\mu_{{\text{G}}}\) :
-
Viscosity of the gas (Pa s)
- \(\mu_{{\text{L}}}\) :
-
Viscosity of the liquid (Pa s)
- \(\rho_{C}\) :
-
Density of the catalyst (kg/m3)
- \(\rho_{{\text{L}}}\) :
-
Density of the liquid (kg/m3)
- \(\rho_{{\text{M}}}\) :
-
Density of the monomer (kg/m3)
- \(\rho_{{\text{P}}}\) :
-
Density of the polymer (kg/m3)
- \(\rho_{{\text{S}}}\) :
-
Density of the solvent (kg/m3)
- \(\rho_{{\text{S,avg}}}\) :
-
Average density of the macroparticle [kg (catalyst + polymer)/m3 macroparticle]
- \(\sigma_{{\text{L}}}\) :
-
Surface tension of liquid (N/m)
References
Chum PS, Swogger KW (2008) Olefin polymer technologies-history and recent progress at the Dow Chemical Company. Prog Polym Sci 33:797–819
Xie T, McAuley KB, Hsu JCC, Bacon DW (1994) Gas phase ethylene polymerization: production processes, polymer properties, and reactor modeling. Ind Eng Chem Res 33:449–479
Khare NP, Seavey KC, Liu YA, Ramanathan S, Lingard S, Chen CC (2002) Steady-state and dynamic modeling of commercial slurry high-density polyethylene (HDPE) processes. Ind Eng Chem Res 41:5601–5618
Zhou Y, Zhang R, Ren H, He X, Liu B, Zhao N, Liu B (2020) Ethylene polymerization over novel organic magnesium based V/Ti bimetallic Ziegler–Natta catalysts. J Organomet Chem 908:121066
Dengfei W, Guoxing Y, Feng G, Wang J, Jiang Y (2018) Progress in technology and catalysts for continuous stirred tank reactor type slurry phase polyethylene processes. Petrol Chem 58:264–273
Ahmadi M, Nekoomanesh M, Arabi H (2010) A simplified comprehensive kinetic scheme for modeling of ethylene/1-butene copolymerization using Ziegler–Natta catalysts. Macromol React Eng 4:135–144
Nassiri H, Arabi H, Hakim S (2012) Kinetic modeling of slurry propylene polymerization using a heterogeneous multi-site type Ziegler–Natta catalyst. React Kinet Mech Catal 105:345–359
Abbasi MR, Shamiri A, Hussain MA (2016) Dynamic modeling and molecular weight distribution of ethylene copolymerization in an industrial gas-phase fluidized-bed reactor. Adv Powder Technol 27:1526–1538
Buls VW, Higgins TL (1970) A particle growth theory for heterogeneous Ziegler polymerization. J Polym Sci Part A-1 Polym Chem 8:1037–1053
Nagel EJ, Kirillov VA, Ray WH (1980) Prediction of molecular weight distributions for high-density polyolefins. Ind Eng Chem Prod Res Dev 19:372–379
Hutchinson RA, Chen CM, Ray WH (1992) Polymerization of olefins through heterogeneous catalysis X: modeling of particle growth and morphology. J Appl Polym Sci 44:1389–1414
Dashti A, Ramazani A (2008) Modeling and simulation of olefin polymerization at microstructure level. Iran J Chem Chem Eng 27:13–22
Touloupides V, Kanellopoulos V, Krallis A, Pladis P, Kiparissides C (2010) Modeling and simulation of particle size distribution in slurry-phase olefin catalytic polymerization industrial loop reactors. Comput Aided Chem Eng 28:43–48
McKenna TFL, Soares JBP (2001) Single particle modelling for olefin polymerization on supported catalysts: a review and proposals for future developments. Chem Eng Sci 56:3931–3949
Alizadeh A, McKenna TFL (2018) Particle growth during the polymerization of olefins on supported catalysts. Part 2: current experimental understanding and modeling progresses on particle fragmentation, growth and morphology development. Macromol React Eng 12:1700027
Schmeal WR, Street JR (1971) Polymerization in expanding catalyst particles. AIChE J 17:1188–1197
Singh D, Merrill RP (1971) Molecular weight distribution of polyethylene produced by Ziegler–Natta catalysts. Macromolecules 4:599–604
Kulkarni S, Mishra V, Bontu NM (2019) A comprehensive model for the micro and meso-scale level olefin polymerization: framework and predictions. Iran Polym J 28:597–609
Najafi M, Parvazinia M, Ghoreishy MHR (2014) Modelling the catalyst fragmentation pattern in relation to molecular properties and particle overheating in olefin polymerization. Polyolefin J 1:77–91
Nouri M, Parvazinia M, Arabi H (2015) Effect of fragment size distribution on reaction rate and molecular weight distribution in heterogeneous olefin polymerization. Iran Polym J 24:437–448
Crabtree JR, Grimsby FN, Nummelin AJ, Sketchley JM (1973) The role of diffusion in the Ziegler polymerization of ethylene. J Appl Polym Sci 17:959–976
Choi KY, Ray WH (1985) Polymerization of olefins through heterogeneous catalysis. II. Kinetics of gas phase propylene polymerization with Ziegler–Natta catalysts. J Appl Polym Sci 30:1065–1081
Casalini T, Visscher F, Tamaddoni M, Nicolaas F, Bertola F, Storti G, Morbidelli M (2018) The effect of residence time distribution on the slurry-phase catalytic ethylene polymerization: an experimental and computational study. Macromol React Eng 12:1700058
Sarkar P, Gupta SK (1991) Modelling of propylene polymerization in an isothermal slurry reactor. Polymer 32:2842–2852
Sarkar P, Gupta SK (1992) Simulation of propylene polymerization: an efficient algorithm. Polymer 33:1477–1485
Sau M, Gupta SK (1993) Modelling of a semibatch polypropylene slurry reactor. Polymer 34:4417–4426
Varshney P, Kunzru D, Gupta SK (2015) Modelling of the riser reactor in a resid fluidised-bed catalytic cracking unit using a multigrain model for an active matrix-zeolite catalyst. Indian Chem Eng 57:115–135
Floyd S, Choi KY, Taylor TW, Ray WH (1986) Polymerization of olefins through heterogeneous catalysis. IV. Modeling of heat and mass transfer resistance in the polymer particle boundary layer. J Appl Polym Sci 31:2231–2265
Fontes CH, Mendes MJ (2005) Analysis of an industrial continuous slurry reactor for ethylene–butene copolymerization. Polymer 46:2922–2932
Han-Adebekun GC, Hamba M, Ray WH (1997) Kinetic study of gas phase olefin polymerization with a TiCl4/MgCl2 catalyst. I. Effect of polymerization conditions. J Polym Sci Part A Polym Chem 35:2063–2074
Soares JBP, McKenna TFL (2012) Polyolefin reaction engineering. Wiley, Weinheim
Soni NJ, Bhagwat SS (2008) Simulation of slurry polymerization of ethylene. Int J Chem React Eng 6:A107
Sun J, Wang H, Tian S, Fan X, Shi Q, Liu Y, Hu X, Yang Y, Wang J, Yang Y (2020) Important mesoscale phenomena in gas phase fluidized bed ethylene polymerization. Particuology 48:116–143
Bhagwat MS, Bhagwat SS, Sharma MM (1994) Mathematical modeling of the slurry polymerization of ethylene: gas-liquid mass transfer limitations. Ind Eng Chem Res 33:2322–2330
Hoe EL, Cozewith C (1994) Effect of diffusion on heterogenous ethylene propylene copolymerization. AIChE J 40:1669–1684
Ha K, Yoo K, Rhee H (2001) Modeling and analysis of a slurry reactor system for heterogeneous olefin polymerization: the effects of hydrogen concentration and initial catalyst size. J Appl Polym Sci 79:2480–2493
Sarkar P, Gupta SK (1992) Steady state simulation of continuous-flow stirred-tank slurry propylene polymerization reactors. Polym Eng Sci 32:732–742
Touloupides V, Kanellopoulos V, Pladis P, Kiparissides C, Mignonvan-Grambezen DP (2010) Modeling and simulation of an industrial slurry-phase catalytic olefin polymerization reactor series. Chem Eng Sci 65:3208–3222
Touloupides V (2014) Catalytic olefin polymerization process modeling: multi-scale approach and modeling guidelines for micro-scale/kinetic modeling. Macromol React Eng 8:508–527
Abbasi MR, Shamiri A, Hussain M (2019) A review on modeling and control of olefin polymerization in fluidized-bed reactors. Rev Chem Eng 35:311–333
Floyd S, Heiskanen T, Taylor TW, Mann GE, Ray WH (1987) Polymerization of olefins through heterogeneous catalysis. VI. Effect of particle heat and mass transfer on polymerization behavior and polymer properties. J Appl Polym Sci 33:1021–1065
Krajakova L, Laskova M, Chmelar J, Jindrova K, Kosek J (2019) Sorption of liquid diluents in polyethylene: comprehensive experimental data for slurry polymerization. Ind Eng Chem Res 58:7037–7043
Gupta SK (2014) Numerical methods for engineers. New Academic Science, London
Calderbank PH, Moo-Young MB (1961) The continuous phase heat and mass-transfer properties of dispersions. Chem Eng Sci 16:39–54
Treybal RE (1980) Mass transfer operations. McGraw-Hill, New York
Bhavaraju SM, Russell TWF, Blanch HW (1978) The design of gas sparged devices for viscous liquid systems. AIChE J 24:454–466
Kawase Y, Hashiguchi N (1996) Gas-liquid mass transfer in external-loop airlift columns with Newtonian and non-Newtonian fluids. Chem Eng J Biochem Eng J 62:35–42
Li J, Tekie Z, Mizan TI, Morsi BI, Maier EE, Singh CPP (1996) Gas-liquid mass transfer in a slurry reactor operating under olefinic polymerization process conditions. Chem Eng Sci 51:549–559
Pladis P, Baltsas A, Meimaroglou D, Kiparissides C (2018) A dynamic simulator for slurry-phase catalytic olefin copolymerization in a series of CSTRs: prediction of distributed molecular and rheological properties. Macromol React Eng 12:1800017
Naderpour N, Vasheghani-Farahani E, Famili MHN, Vatankhah M (2010) Kinetics of slurry and gas phase polymerizations of ethylene using a novel heterogeneous Ziegler–Natta catalyst of specific morphology. Iran Polym J 19:895–906
Wilke CR, Chang P (1955) Correlation of diffusion coefficients in dilute solutions. AIChE J 1:264–270
Rumble JR, Lide DR, Bruno TJ (1991) CRC handbook of chemistry and physics. CRC, Boca Raton
Soares JBP, Romero J (2017) A monte carlo method to quantify the effect of reactor residence time distribution on polyolefins made with heterogeneous catalysts. Part I. Catalyst/polymer particle size distribution effects. Macromol React Eng 12:1700038
Keii T, Suzuki E, Tamura M, Doi Y (1981) MMI Int Symp on transition metal catalyzed polymerization: unsolved problems. Midland, p 37
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thakur, A.K., Gupta, S.K. & Chaudhari, P. Modeling and simulation of an industrial slurry phase ethylene polymerization reactor: effect of reactor operating variables. Iran Polym J 29, 811–825 (2020). https://doi.org/10.1007/s13726-020-00840-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-020-00840-6