Abstract
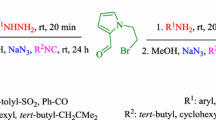
A novel series of oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indoles were synthesized via a two-step pathway. In the first step, Ugi-four-component condensation of 2-formylindole, amines, (E)-4-alkoxy-4-oxobut-2-enoic acids, and isocyanides gave the corresponding Ugi-adducts. This adduct underwent intramolecular hydroamination in the presence of K2CO3 in CH3CN at room temperature to afford diastereoselective synthesis of a range of oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indoles. A comparison of experimentally observed CD and UV–visible spectra with the theoretical DFT calculated ECD spectra was used to predict the major diastereomer.





Similar content being viewed by others
References
B.M. Trost, Science 254, 1471 (1991)
L.F. Tietze, Chem. Rev. 96, 115 (1996)
P.A. Wender, S.T. Handy, D.L. Wright, Chem. Ind. 765, 767 (1997)
S.L. Schreiber, Science 287, 1964 (2000)
A. Dömling, I. Ugi, Angew. Chem. Int. Ed. 39, 3168 (2000)
A. Domling, Chem. Rev. 106, 17 (2006)
J.D. Sunderhaus, S.F. Martin, Chem. Eur. J. 15, 1300 (2009)
B.B. Touré, D.G. Hall, Chem. Rev. 109, 4439 (2009)
B. Ganem, Acc. Chem. Res. 42, 463 (2009)
A. Váradi, T.C. Palmer, R.N. Dardashti, S. Majumdar, Molecules 21, 19 (2016)
N.A. Afagh, A.K. Yudin, Angew. Chem. Int. Ed. 49, 262 (2010)
U.K. Sharma, N. Sharma, D.D. Vachhania, E.V. Van der Eycken, Chem. Soc. Rev. 44, 1836 (2015)
I. Ugi, R. Meyr, U. Fetzer, C. Steinbrückner, Angew. Chem. 71, 386 (1959)
I. Ugi, C. Steinbrückner, Angew. Chem. 72, 2671960 (1959)
J. Isaacson, Y. Kobayashi, Angew. Chem. Int. Ed. 48, 1845 (2009)
A. Dömling, B. Beck, U. Eichelberger, S. Sakamuri, S. Menon, Q.-Z. Chen, Y. Lu, L.A. Wessjohann, Angew. Chem. Int. Ed. 45, 7235 (2006)
M.J. Thompson, B. Chen, J. Org. Chem. 74, 7084 (2009)
W. Erb, J.-P. Neuville, L. Zhu, J. Org. Chem. 74, 3109 (2009)
D.G. Riveraa, L.A. Wessjohann, J. Am. Chem. Soc. 131, 3721 (2009)
D. Coffinier, L. El Kaim, L. Grimaud, Org. Lett. 11, 995 (2009)
L.A. Wessjohann, D.G. Rivera, O.E. Vercillo, Chem. Rev. 109, 796 (2009)
G. Cuny, M. Bois-Choussy, J. Zhu, J. Am. Chem. Soc. 126, 14475 (2004)
U.K. Sharma, N. Sharma, D.D. Vachhani, E.V. Van der Eycken, Chem. Soc. Rev. 44, 1836 (2015)
A. Aygun, U. Pindur, Curr. Med. Chem. 10, 1113 (2003)
F.R. deSa´Alves, E.J. Barreiro, C.A. Fraga, Med. Chem. 9, 782 (2009)
M. Ishikura, K. Yamada, T. Abe, Nat. Prod. Rep. 27, 1630 (2010)
R.J. Sundberg, The Chemistry of Indoles (Academic Press, New York, 1970)
R.J. Sundberg, in Comprehensive Heterocyclic Chemistry, ed. by A.R. Katritzky, C.W. Rees (Pergamon Press, Oxford, 1984), Vol. 4
J.A. Joule, E.J. Thomas, ed., Science of Synthesis, Houben-Weyl Methods of Molecular Transformations, vol. 10 (George Thieme Verlag, Stuttgart, 2000), Chap. 10
G.W. Gribble, in Comprehensive Heterocyclic Chemistry II, vol. 2, ed. by A.R. Katritzky, C.W. Ress, E.F.V. Scriven, C.W. Bird (Pergamon Press, Oxford, 1996), p. 207
R.J. Sundberg, Indoles, (Academic Press, London, 1996)
D. Zhou, P. Zhou, D.A. Evrard, K. Meagher, M. Webb, B.L. Harrison, D.M. Huryn, J. Golembieski, G.A. Hornby, L.E. Schechter, D.L. Smith, T.H. Andree, R.E. Mewshaw, Bioorg. Med. Chem. 16, 6707 (2008)
J.D. Williams, S.T. Nguyen, S. Gu, X. Ding, M.M. Butler, T.F. Tashjian, T.J. Opperman, T.L. Bowlin, R.G. Panchal, S. Bavari, N.P. Peet, D.T. Moir, Bioorg. Med. Chem. Lett. 21, 7790 (2013)
M.Z. Zhang, Q. Chen, G.F. Yang, Eur. J. Med. Chem. 89, 421 (2015)
M. Shiri, Chem. Rev. 112, 3508 (2012)
M.M. Heravi, T. Alishiri, Adv. Heterocycl. Chem. 113, 1 (2014)
M.M. Heravi, B. Talaei, Adv. Heterocycl. Chem. 113, 143 (2014)
M.M. Heravi, S. Khaghaninejad, M. Mostofi, Adv. Heterocycl. Chem. 112, 1 (2014)
M.M. Heravi, S. Khaghaninejad, N. Nazari, Adv. Heterocycl. Chem. 112, 183 (2014)
M.M. Heravi, B. Talaei, Adv. Heterocycl. Chem. 114, 147 (2015)
M.M. Heravi, V.F. Vavsari, Adv. Heterocycl. Chem. 114, 77 (2015)
M.M. Heravi, V. Zadsirjan, Adv. Heterocycl. Chem. 117, 261 (2015)
M.M. Heravi, B. Talaei, Adv. Heterocycl. Chem. 118, 195 (2016)
M. Shiri, M. Ranjbar, Z. Yasaei, F. Zamanian, B. Notash, Org. Biomol. Chem. 15, 10073 (2017)
M. Shiri, Z. Faghihi, H.A. Oskouei, M.M. Heravi, B. Notash, Sh. Fazelzadeh, RSC Adv. 6, 92235 (2016)
S. Sadjadi, M.M. Heravi, N. Nazari, RSC Adv. 6, 53203 (2016)
F. Nemati, M.M. Heravi, A. Elhampour, RSC Adv. 5, 45775 (2015)
M.M. Heravi, E. Hashemi, Y.S. Beheshtiha, K. Kamjou, M. Toolabi, N. Hosseintash, J. Mol. Catal. A Chem. 392, 173 (2014)
M.M. Heravi, F. Mousavizadeh, N. Ghobadi, M. Tajbakhsh, Tetrahedron Lett. 55, 1226 (2014)
M.M. Heravi, S. Moghimi, Tetrahedron Lett. 53, 392 (2012)
M.M. Heravi, S. Sadjadi, N. Mokhtari Haj, H.A. Oskooie, F.F. Bamoharram, Catal. Commun. 10, 1643 (2009)
M.M. Heravi, M. Daraie, Molecules 21, 441 (2016)
A. Rezvanian, M.M. Heravi, Z. Shaabani, M. Tajbakhsh, Tetrahedron 73, 2017 (2009)
M. Shiri, Z. Bozorgpour-Savadjani, J. Iran. Chem. Soc. 12, 389 (2015)
M. Shiri, S.Z. Mirpour-Marzoni, Z. Bozorgpour-Savadjani, B. Soleymanifard, H.G. Kruger, Monatsh. Chem. 145, 1947 (2014)
S.M. hiri, B. Farajpour, Z. Bozorgpour-Savadjani, S.A. Shintre, N.A. Koorbanally, H.G. Kruger, B. Notash, Tetrahedron 71, 5531 (2015)
B. Soleymanifard, M.M. Heravi, M. Shiri, M.A. Zolfigol, M. Rafiee, H.G. Kruger, T. Naicker, F. Rasekhmanesh, Tetrahedron Lett. 53, 3546 (2012)
V. Zadsirjan, M. Shiri, M.M. Heravi, T. Hosseinnejad, S.A. Shintre, N.A. Koorbanally, Res. Chem. Intermed. 43, 2119 (2017)
K. Maruoka, M. Akakura, S. Saito, T. Ooi, H. Yamamoto, J. Am. Chem. Soc. 116, 6153 (1994)
H.J. Zhu, Organic Stereochemistry: Experimental and Computational Methods (2015), p. 163
P.J. Stephens, N. Harada, Chirality 22, 229 (2010)
S.S. Makhathini, S.K. Das, T. Singh, P.I. Arvidsson, H.G. Kruger, H. Gunosewoyo, T. Govender, T. Naicker, Arkivoc 3, 134 (2016)
G. Scalmani, M.J. Frisch, B. Mennucci, J. Tomasi, R. Cammi, V. Barone, J. Chem. Phys. 124, 94107 (2006)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, V.G. Zakrzewski, J.A. Montgomery, R.E. Stratmann, J.C. Burant, S. Dapprich, J.M. Millam, A.D. Daniels, K.N. Kudin, M.C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G.A. Petersson, P.Y. Ayala, Q. Cui, K. Morokuma, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J. Cioslowski, J.V. Ortiz, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P.M.W. Gill, B.G. Johnson, W. Chen, M.W. Wong, J.L. Andres, M. Head-Gordon, E.S. Replogle, J.A. Pople, Gaussian 98 (Revision A.1). (Gaussian, Inc., Pittsburgh PA, 1998)
R.G. Parr, W. Yang, Density-Functional Theory of Atoms and Molecules (Oxford University Press, Oxford, 1989)
R.M. Dickson, A.D. Becke, J. Phys. Chem. 100, 16105 (1196)
K. Wolinski, J.F. Hilton, P.J. Pulay, J. Am. Chem. Soc. 112, 825 (1990)
Acknowledgements
The authors would like to thank the Alzahra University and Iran National Science Foundation (INSF) for financial support. We are also very grateful for Prof. Adolf Gogoll and Dr. Sandra Olsson from Uppsala University (Uppsala, Sweden) for doing experimental ECD and UV–visible spectroscopy.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shiri, M., Heravi, M.M., Zadsirjan, V. et al. Highly regio- and diastereoselective synthesis of oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indoles, based on a post-Ugi condensation: joint experimental and computational study. J IRAN CHEM SOC 16, 1517–1526 (2019). https://doi.org/10.1007/s13738-019-01632-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01632-3