Abstract
The goal of synthetic biology is to create artificial organisms. To achieve this it is essential to understand what life is. Metabolism-replacement systems, or (M, R)-systems, constitute a theory of life developed by Robert Rosen, characterized in the statement that organisms are closed to efficient causation, which means that they must themselves produce all the catalysts they need. This theory overlaps in part with other current theories, including autopoiesis, the chemoton, and autocatalytic sets, all of them invoking some idea of closure. A simple model of an (M, R)-system has been implemented in the computer, and behaves in ways that may shed light on the requirements for a prebiotic self-organizing system. In addition to a trivial steady state in which nothing happens, it can establish a non-trivial steady state in which all intermediates have finite concentrations, with their rates of degradation balanced by their rates of synthesis. The system can be regenerated from the set of food components plus a single intermediate, and maintain itself in that state indefinitely, despite continuous degradation. At the very low compartment volumes that may have existed in prebiotic conditions, for example in cavities in minerals, or in micelles formed by simple amphiphiles, statistical fluctuations in the numbers of molecules need to be taken into account. With the stochastic approach there is no non-trivial steady state in strict mathematical terms, because the system will always collapse to the trivial state after sufficient time. However, the average time before collapse is so long for volumes greater than \(10^{-19}\;{\textsc{l}}\) (much smaller than the volume of the order of \(10^{-15}\;{\textsc{l}}\) for a typical bacterial cell) that for practical purposes the self-maintaining state of non-null concentrations becomes significant, recalling the situation of bistability that is observed in deterministic analysis. In turn, there exists a minimum size below which the self-organizing system cannot maintain itself on chemically relevant time scales. The value of the critical volume depends on the particular concentrations and rate constants assumed, but the principle could apply generally.



Similar content being viewed by others
Notes
This sort of approximation was useful when computer time and storage were orders of magnitude more expensive than they are now. Today one would simply store the entire table, or calculate the appropriate value to any desired precision with the proper statistical theory.
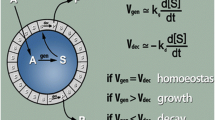
It is an everyday observation that the chemical output of any organism is different from the food taken in, and it should be obvious from thermodynamic considerations that that must be the case.
The collapse would not be totally irreversible if we allow uncatalyzed production of minimal amounts of intermediates: in this case the system could regain the non-trivial steady state if all of the degradation rate constants were sufficiently low.
For example, for k = 0.1 s−1, a starting concentration of STU of at least \(0.135\; \text{m}{\textsc{m}}\) would allow the whole system to construct itself.
This is much smaller than the volume of more than \(10^{-15}\; {\textsc{l}}\; (1\; \upmu\text{m}^3\)) for a bacterium such as Escherichia coli.
In their recent paper on osmotic structures resembling Leduc’s osmotic gardens, Barge et al. (2012) do not give estimates of the internal volumes of their structures. They have informed us (Barge, personal communication, 8 March 2012) that although these volumes are very difficult to estimate with any accuracy they are certainly orders of magnitude larger than the volumes we are considering here.
It is easy to forget that supposedly improbable events occur very frequently. In a human population of 109 individuals the probability that one of them will experience an event of probability 10−9 at any particular moment is close to certainty.
This is just one illustration of the point that all current theories of life lack some features that an ideal theory ought to have (Letelier et al. 2011). On the other hand the chemical nature of the catalysts—absolutely necessary for the specificity that any self-organizing set of reactions must have—is too vague in autopoiesis and the chemoton to be satisfactory.
References
Barge LM, Doloboff IJ, White LM, Stucky GD, Russell MJ, Kanik I (2012) Characterization of iron–phosphate–silicate chemical garden structures. Langmuir 28(8):3714–3721 doi:10.1021/la203727g
Brenner K, You L, Arnold FH (2008) Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 26(9):483–489 doi:10.1016/j.tibtech.2008.05.004
Briggs DE (1962) Simplification of assay of enzymes using non-linear reaction-progress graphs. Enzymologia 24(2):97–104
Briones C, Stich M, Manrubia SC (2009) The dawn of the RNA World: toward functional complexity through ligation of random RNA oligomers. RNA Publ RNA Soc 15(5):743–749 doi:10.1261/rna.1488609
Cárdenas ML, Letelier JC, Gutierrez C, Cornish-Bowden A, Soto-Andrade J (2010) Closure to efficient causation, computability and artificial life. J Theor Biol 263(1):79–92 doi:10.1016/j.jtbi.2009.11.010
Chemero A, Turvey MT (2006) Complexity and closure to efficient cause. In: Ruiz-Mirazo K, Barandiaran R (eds) ALIFE X: workshop on artificial autonomy. MIT Press, Cambridge, MA, pp 13–18
Chen IA, Walde P (2010) From self-assembled vesicles to protocells. Cold Spring Harbor Perspect. Biol. 2(7) doi:10.1101/cshperspect.a002170
Chiarabelli C, Stano P, Anella F, Carrara P, Luisi P (2012) Approaches to chemical synthetic biology. FEBS Lett 586(15):2138–2145 doi:10.1016/j.febslet.2012.01.014
Cornish-Bowden A, Cárdenas ML, Letelier JC, Soto-Andrade J (2007) Beyond reductionism: metabolic circularity as a guiding vision for a real biology of systems. Proteomics 7(6):839–845 doi:10.1002/pmic.200600431
de la Mettrie JJO (1748) L’Homme machine. Elie Luzac, Leyden
Eigen M, Schuster P (1977) The hypercycle—a principle of natural self-organization. A. Emergence of the hypercycle. Naturwissenschaften 64(11):541–565 doi:10.1007/BF00450633
Gánti T (2003) The principles of life. Oxford University Press, Oxford
Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, Vashee S, Krishnakumar R, Assad-Garcia N, Andrews-Pfannkoch C, Denisova EA, Young L, Qi ZQ, Segall-Shapiro TH, Calvey CH, Parmar PP, Hutchison III, CA, Smith HO, Venter JC (2010) Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329(5987): 52–56
Gillespie DT (1976) A general method for numerically simulating stochastic time evolution of coupled chemical-reactions. J Comput Phys 22(4):403–434 doi:10.1016/0021-9991(76)90041-3
Gillespie DT (1977) Exact stochastic simulation of coupled chemical-reactions. J Phys Chem 81(25):2340–2361 doi:10.1021/j100540a008
Hanczyc MM, Fujikawa SM, Szostak JW (2003) Experimental models of primitive cellular compartments: encapsulation, growth, and division. Science 302(5645):618–622 doi:10.1126/science.1089904
Hoops S, Sahle S, Gauges R, Lee C, Pahle J, Simus N, Singhal M, Xu L, Mendes P, Kummer U (2006) COPASI—a Complex pathway simulator. Bioinformatics 22(24):3067–3074 doi:10.1093/bioinformatics/btl485
Hordijk W, Fontanari JF (2003) Catalytic reaction sets, decay, and the preservation of information. In: Hexmoor H (ed) International conference on integration of knowledge intensive multi-agent systems: KIMAS’03: Modeling, exploration, and engineering. IEEE, New York, pp 133–138 doi:10.1109/KIMAS.2003.1245035
Kauffman SA (1986) Autocatalytic sets of proteins. J Theor Biol 119(1):1–24 doi:10.1016/S0022-5193(86)80047-9
Leduc S (1912) La Biologie Synthétique. Poinat, Paris
Letelier JC, Cárdenas ML, Cornish-Bowden A (2011) From L’Homme machine to metabolic closure: steps towards understanding life. J Theor Biol 286:100–113 doi:10.1016/j.jtbi.2011.06.033
Letelier JC, Kuboyama T, Yasuda TH, Cárdenas ML, Cornish-Bowden A (2005) A self-referential equation, f(f) = f, obtained using the theory of (M, R) systems: overview and applications. In: Anai H, Horimoto K (eds) Algebraic biology. Universal Academy Press, Tokyo, pp 115–126
Letelier JC, Soto-Andrade J, Guíñez Abarzua F., Cornish-Bowden A., Cárdenas ML (2006) Organizational invariance and metabolic closure: analysis in terms of (M, R) systems. J Theor Biol 238(4):949–961 doi:10.1016/j.jtbi.2005.07.007
Luisi PL (2006) The emergence of life. Cambridge University Press, Cambridge
Martin W, Russell MJ (2003) On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Phil Trans R Soc B 358(1429):59–83 doi:10.1098/rstb.2002.1183
Maturana H, Varela F (1980) Autopoiesis and cognition: the realisation of the living. D. Reidel Publishing Company, Dordrecht
Mill JS (1846) A system of logic. Harper & Brothers, New York, pp 211
Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, Dulay GP, Hua KL, Ankoudinova I, Cost GJ, Urnov FD, Zhang HS, Holmes MC, Zhang L, Gregory PD, Rebar EJ (2011) A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 29(2):143–148 doi:10.1038/nbt.1755
Morán F, Moreno A, Minch E, Montero F (1996) Further steps towards a realistic description of the essence of life. In: Langton CG, Shimohara K (eds) Artificial life V. MIT Press, Cambridge, MA, pp 255–263
Piedrafita G, Montero F, Morán F, Cárdenas ML, Cornish-Bowden A (2010) A simple self-maintaining metabolic system: robustness, autocatalysis, bistability. PLoS Comput Biol 6(8):e1000,872 doi:10.1371/journal.pcbi.1000872
Piedrafita G, Cornish-Bowden A, Morán F, Montero F (2012a) Size matters: influence of stochasticity on the self-maintenance of a simple model of metabolic closure. J Theor Biol 300:143–151
Piedrafita G, Ruiz-Mirazo K, Monnard PA, Cornish-Bowden A, Montero F (2012b) Viability conditions for a compartmentalized protometabolic system: a semi-empirical approach. PLoS ONE 7:e39,480
Rosen R (1971) Some realizations of (M, R)-systems and their interpretation. Bull Math Biophys 33(3):303–319 doi:10.1007/BF02476776
Rosen R (1973) On the dynamical realization of (M, R)-systems. Bull Math Biol 35(1-2):1–9
Rosen R (1991) Life itself. Columbia University Press, New York
Ruiz-Mirazo K, Piedrafita G, Ciriaco F, Mavelli F (2011) Stochastic simulations of mixed-lipid compartments: from self-assembling vesicles to self-producing protocells. Adv Exp Med Biol 696:689–696
Sánchez-Izquierdo F, Blanco P, Busqué F, Alibés R, de March P, Figueredo Galimany M, Font J, Parella T (2007) Total synthesis of the putative structure of stemonidine: the definitive proof of misassignment. Org Lett 9(9):1769–1772 doi:10.1021/ol070486p
Schrödinger E (1944) What is life? Cambridge University Press, Cambridge, UK
Smith MT, Cornish-Bowden A, Briggs DE (1979) Determination of barley α-amylase activity. J Inst Brew 85(4):157–159
Szathmáry E (2007) Coevolution of metabolic networks and membranes: the scenario of progressive sequestration. Philos Trans R Soc 362(1486):1781–1787 doi:10.1098/rstb.2007.2070
Szostak JW, Bartel DP, Luisi PL (2001) Synthesizing life. Nature 409(6818):387–390
Walde P (2006) Surfactant assemblies and their various possible roles for the origin(s) of life. Orig Life Evol Biosphere 36(2):109–150 doi:10.1007/s11084-005-9004-3
Acknowledgements
This work was supported by grant BFU2009-12895-C02-02 (Ministerio de Ciencia e Innovación, Spain). GP acknowledges support from PhD scholarship FPU (Formación del Profesorado Universitario, Ministry of Education, Spain). AC-B and MLC were supported by the Centre National de la Recherche Scientifique.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cornish-Bowden, A., Piedrafita, G., Morán, F. et al. Simulating a Model of Metabolic Closure. Biol Theory 8, 383–390 (2013). https://doi.org/10.1007/s13752-013-0132-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13752-013-0132-0