Abstract
BACKGROUND:
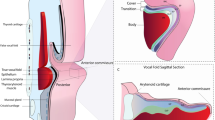
The vocal cord tissue consists of three anatomical layers from the surface to deep inside: the epithelium that contains almost no collagen, the lamina propria that is composed of abundant collagen, and the vocalis muscle layer. It is clinically important to visualize the tissue microstructure using a non-invasive method, especially in the case of vocal cord nodules or cancer, since histological changes in each layer of the vocal cord cause changes in the voice. Polarization-sensitive optical coherence tomography (PS-OCT) enables phase retardation measurement to evaluate birefringence of tissue with varied organization of collagen fibers in different tissue layers. Therefore, PS-OCT can visualize structural changes between normal and abnormal vocal cord tissue.
METHOD:
A rabbit laryngeal tumor model with different stages of tumor progression was investigated ex-vivo by PS-OCT. A phase retardation slope-based analysis, which quantifies the birefringence in different layers, was conducted to distinguish the epithelium, lamina propria, and muscle layers.
RESULTS:
The PS-OCT images showed a gradual decrease in birefringence from normal tissue to advanced tumor tissue. The quantitative analysis provided a more detailed comparison among different stages of the rabbit laryngeal tumor model, which was validated by the corresponding histological findings.
CONCLUSION:
Differences in tissue birefringence was evaluated by PS-OCT phase retardation measurement. It is also possible to indirectly infer the dysplastic changes based on the mucosal and submucosal alterations.





Similar content being viewed by others
References
Subotić R, Vecerina S, Krajina Z, Hirano M, Kurita S. Histological structure of vocal fold lamina propria in foetal larynx. Acta Otolaryngol. 1984;97:403–6
Kwon SK, Lee EK, Kim HB, Song JJ, Cho CG, Park SW. Quantitative evaluation of laryngeal function in glottal insufficiency animal model for tissue engineering approach. Tissue Eng Regen Med. 2013;10:322–8.
Wong BJ, Jackson RP, Guo S, Ridgway JM, Mahmood U, Su J, et al. In vivo optical coherence tomography of the human larynx: normative and benign pathology in 82 patients. Laryngoscope. 2005;115:1904–11.
Armstrong WB, Ridgway JM, Vokes DE, Guo S, Perez J, Jackson RP, et al. Optical coherence tomography of laryngeal cancer. Laryngoscope. 2006;116:1107–13.
Just T, Pau HW, Lankenau E, Hüttmann G. OCT in the field of laryngology: further perspectives. Photonic therapeutics and diagnostics VII. Proc SPIE Int Soc Opt Eng. 2011;7883:788831W.
Ju MJ, Hong YJ, Makita S, Lim Y, Kurokawa K, Duan L, et al. Advanced multi-contrast Jones matrix optical coherence tomography for Doppler and polarization sensitive imaging. Opt Express. 2013;21:19412–36
Pierce MC, Strasswimmer J, Hyle Park B, Cense B, De Boer JF. Birefringence measurements in human skin using polarization-sensitive optical coherence tomography. J Biomed Opt. 2004;9:287–91
Kim KH, Pierce MC, Maguluri G, Park BH, Yoon SJ, Lydon M, et al. In vivo imaging of human burn injuries with polarization-sensitive optical coherence tomography. J Biomed Opt. 2012;17:066012.
Li X, Martin S, Pitris C, Ghanta R, Stamper DL, Harman M, et al. High-resolution optical coherence tomographic imaging of osteoarthritic cartilage during open knee surgery. Arthritis Res Ther. 2005;7:R318–23.
Fleischhauer F, Schulz-Hildebrandt H, Bonin T, Huttmann G. Polarization-sensitive optical coherence tomography on different tissues samples for tumor discrimination. In: Student Conference Medical Engineering Science. 2013.
Duan L, Yamanari M, Yasuno Y. Automated phase retardation oriented segmentation of chorio-scleral interface by polarization sensitive optical coherence tomography. Opt Express. 2012;20:3353–66
Zhou X, Ju MJ, Huang L, Tang S. Correlation between polarization sensitive optical coherence tomography and SHG microscopy in articular cartilage. In: Optical coherence tomography and coherence domain optical methods in biomedicine XXI. Int Soc for Optics and Photonics. 2017;10053:1005319.
Kim KH, Burns JA, Bernstein JJ, Maguluri GN, Park BH, de Boer JF. In vivo 3D human vocal fold imaging with polarization sensitive optical coherence tomography and a MEMS scanning catheter. Opt Express. 2010;18:14644–53.
Burns JA. Optical coherence tomography: imaging the larynx. Curr Opin Otolaryngol Head Neck Surg. 2012;20:477–81.
National Research Council (US) Institute for Laboratory Animal Research, NIH Guide for the Care and Use of Laboratory Animals. Washinton: National Academic Press; 1996.
Shin YS, Lee JS, Choi JW, Min BH, Chang JW, Lim JY, et al. Transplantation of autologous chondrocytes seeded on a fibrin/hyaluronic acid composite gel into vocal fold in rabbits: preliminary results. Tissue Eng Regen Med. 2012;9:203–8.
Zhou X, Oak CH, Ahn YC, Kim SW, Tang S. Investigation in clinical potential of polarization sensitive optical coherence tomography in laryngeal tumor model study. In: Optical coherence tomography and coherence domain optical methods in biomedicine XXII. International Society for Optics and Photonics. 2018;10483:1048339.
Strasswimmer J, Pierce MC, Park BH, Neel V, de Boer JF. Polarization-sensitive optical coherence tomography of invasive basal cell carcinoma. J Biomed Opt. 2004;9:292–8.
Oak CH, Ahn YC, Nam SJ, Jung MH, Hwang SS, Chae YG, et al. Multimodal imaging using optical coherence tomography and endolaryngeal ultrasonography in a new rabbit VX2 laryngeal cancer model. Lasers Surg Med. 2015;47:704–10.
Acknowledgements
This study was supported by a grant from the National Research Foundation of Korea (NRF) (2017R1D1A1B03035048, 2019M3E5D1A02070860, 2019M3E5D1A02070865, 2019M3E5D1A02070866).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUS) in Kosin University College of Medicine (IACUC approval No. KMAP 15–07).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xin, Z., Kim, S.W., Oak, C. et al. Investigation of the Clinical Potential of Polarization-Sensitive Optical Coherence Tomography in a Laryngeal Tumor Model. Tissue Eng Regen Med 18, 81–87 (2021). https://doi.org/10.1007/s13770-020-00323-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00323-y