Abstract
Background:
Angiogenesis and vasculogenesis are essential processes for successful tissue regeneration in tissue engineering and regenerative medicine. The adipose-derived stromal vascular fraction (SVF) is not only a source of adipose stem cells (ASC) but also a suitable source of microvascular endothelial cells because it is a rich capillary network. So, we propose a new hypothesis for isolating adipose-derived human microvascular endothelial cells (HMVEC-A) from the SVF and developed a dual isolation system that isolates two cell types from one tissue.
Method:
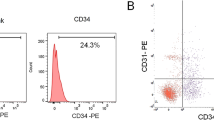
To isolate HMVEC-A, we analyzed the supernatant discarded when ASC is isolated from the adipose-derived SVF. Based on this analysis, we assumed that the SVF adherent to the bottom of the culture plate was divided into two fractions: the stromal fraction as the ASC-rich fraction, and the vascular fraction (VF) as the endothelial cells-rich fraction floating in the culture supernatant. VF isolation was optimized and the efficiency was compared, and the endothelial cells characteristics of HMVEC-A were confirmed by flow cytometric analysis, immunocytochemistry (ICC), a DiI-acetylated low-density lipoprotein (DiI-Ac-LDL) uptake, and in vitro tube formation assay.
Results:
Consistent with the hypothesis, we found a large population of HMVEC-A in the VF and isolated these HMVEC-A by our isolation method. Additionally, this method had higher yields and shorter doubling times than other endothelial cells isolation methods and showed typical morphological and phenotypic characteristics of endothelial cells.
Conclusion:
Cells obtained by the method according to our hypothesis can be applied as a useful source for studies such as tissue-to-tissue networks, angiogenesis and tissue regeneration, patient-specific cell therapy, and organoid chips.






Similar content being viewed by others
References
Marrella A, Lee TY, Lee DH, Karuthedom S, Syla D, Chawla A, et al. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater Today (Kidlington). 2018;21:362–76.
Sarker MD, Naghieh S, Sharma NK, Chen X. 3D biofabrication of vascular networks for tissue regeneration: a report on recent advances. J Pharm Anal. 2018;8:277–96.
Rademakers T, Horvath JM, van Blitterswijk CA, LaPointe VLS. Oxygen and nutrient delivery in tissue engineering: approaches to graft vascularization. J Tissue Eng Regen Med. 2019;13:1815–29.
Mittal R, Woo FW, Castro CS, Cohen MA, Karanxha J, Mittal J, et al. Organ-on-chip models: implications in drug discovery and clinical applications. J Cell Physiol. 2019;234:8352–80.
Collins SD, Yuen G, Tu T, Budzinska MA, Spring K, Bryant K, et al. In vitro models of the liver: disease modeling, drug discovery and clinical applications. In: Tirnitz-Parker JEE, editor. Hepatocellular carcinoma. Brisbane (AU): Codon Publications. 2019. Chapter 3.
Olgasi C, Talmon M, Merlin S, Cucci A, Richaud-Patin Y, Ranaldo G, et al. Patient-specific iPSC-derived endothelial cells provide long-term phenotypic correction of hemophilia A. Stem Cell Reports. 2018;11:1391–406.
Williams IM, Wu JC. Generation of endothelial cells from human pluripotent stem cells. Arterioscler Thromb Vasc Biol. 2019;39:1317–29.
Sharath SS, Ramu J, Nair SV, Iyer S, Mony U, Rangasamy J. Human adipose tissue derivatives as a potent native biomaterial for tissue regenerative therapies. Tissue Eng Regen Med. 2020;17:123–40.
Chun SY, Lim JO, Lee EH, Han MH, Ha YS, Lee JN, et al. Preparation and characterization of human adipose tissue-derived extracellular matrix, growth factors, and stem cells: a concise review. Tissue Eng Regen Med. 2019;16:385–93.
Williams SK, Wang TF, Castrillo R, Jarrell BE. Liposuction-derived human fat used for vascular graft sodding contains endothelial cells and not mesothelial cells as the major cell type. J Vasc Surg. 1994;19:916–23.
Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–26.
Lee SJ, Lee CR, Kim KJ, Ryu YH, Kim E, Han YN, et al. Optimal condition of isolation from an adipose tissue-derived stromal vascular fraction for the development of automated systems. Tissue Eng Regen Med. 2020;17:203–8.
Nguyen A, Guo J, Banyard DA, Fadavi D, Toranto JD, Wirth GA, et al. Stromal vascular fraction: a regenerative reality? Part 1: current concepts and review of the literature. J Plast Reconstr Aesthet Surg. 2016;69:170–9.
Varma MJ, Breuls RG, Schouten TE, Jurgens WJ, Bontkes HJ, Schuurhuis GJ, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16:91–104.
Dykstra JA, Facile T, Patrick RJ, Francis KR, Milanovich S, Weimer JM, et al. Concise review: fat and furious: harnessing the full potential of adipose-derived stromal vascular fraction. Stem Cells Transl Med. 2017;6:1096–108.
Ehrlund A, Acosta JR, Björk C, Hedén P, Douagi I, Arner P, et al. The cell-type specific transcriptome in human adipose tissue and influence of obesity on adipocyte progenitors. Sci Data. 2017;4:170164.
Ramakrishnan VM, Boyd NL. The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Eng Part B Rev. 2018;24:289–99.
Bi H, Li H, Zhang C, Mao Y, Nie F, Xing Y, et al. Stromal vascular fraction promotes migration of fibroblasts and angiogenesis through regulation of extracellular matrix in the skin wound healing process. Stem Cell Res Ther. 2019;10:302.
Dobke M, Peterson DR, Mattern RH, Arm DM, Li WW. Microvascular tissue as a platform technology to modify the local microenvironment and influence the healing cascade. Regen Med. 2020;15:1313–28.
Kim JN, Lee JH, Oh DY, Yoo G, Jeon YJ, Moon SH, et al. Simple, and effective isolation and differentiation of endothelial cells and their progenitor cells from human fat tissue. Tissue Eng Regen Med. 2011;8:A37-43.
Ilesanmi AO. A novel way of harvesting a large population of endothelial cells for clinical and experimental use. Biomed Res Rev. 2018. https://doi.org/10.15761/BRR.1000120.
Schlie S, Gruene M, Dittmar H, Chichkov BN. Dynamics of cell attachment: adhesion time and force. Tissue Eng Part C Methods. 2012;18:688–96.
Sekiguchi H, Ii M, Jujo K, Yokoyama A, Hagiwara N, Asahara T. Improved culture-based isolation of differentiating endothelial progenitor cells from mouse bone marrow mononuclear cells. PLoS One. 2011;6:e28639.
Haynes BA, Huyck RW, James AJ, Carter ME, Gaafar OU, Day M, et al. Isolation, expansion, and adipogenic induction of CD34+CD31+ endothelial cells from human omental and subcutaneous adipose tissue. J Vis Exp. 2018;17:57804.
Van Pham P, Vu NB, Nguyen HT, Phan NK. Isolation of endothelial progenitor cells from human adipose tissue. BMRAT. 2016;3:645–52.
Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. 2017;8:145.
Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci. 2000;97:3422–7.
Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59:1075–88.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28.
Corvera S, Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta. 2014;1842:463–72.
Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88-97.
Freiman A, Shandalov Y, Rozenfeld D, Shor E, Segal S, Ben-David D, et al. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res Ther. 2016;7:5.
Gharibi B, Hughes FJ. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med. 2012;1:771–82.
Cribbs SK, Martin GS, Rojas M. Monitoring of endothelial dysfunction in critically ill patients: the role of endothelial progenitor cells. Curr Opin Crit Care. 2008;14:354–60.
Pill K, Melke J, Muhleder S, Pultar M, Rohringer S, Priglinger E, et al. Microvascular networks from endothelial cells and mesenchymal stromal cells from adipose tissue and bone marrow: a comparison. Front Bioeng Biotechnol. 2018;6:156.
Pill K, Hofmann S, Redl H, Holnthoner W. Vascularization mediated by mesenchymal stem cells from bone marrow and adipose tissue: a comparison. Cell Regen. 2015;4:8.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2017M3A9E2060428) and by a Ministry of Trade Industry and Energy of Korea (10063334).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ryu, Y.H., Moon, SH., Kim, K.J. et al. A Novel Hypothesis and Characterization to Isolate Microvascular Endothelial Cells Simultaneously with Adipose-Derived Stem Cells from the Human Adipose-Derived Stromal Vascular Fraction. Tissue Eng Regen Med 18, 429–440 (2021). https://doi.org/10.1007/s13770-021-00332-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00332-5