Abstract
BACKGROUND:
In vitro follicular maturation (IVFM) of ovarian follicles is an emerging option for fertility preservation. Many paracrine factors and two-dimensional or three-dimensional (3D) environments have been used for optimization. However, since most studies were conducted using the murine model, the physiological differences between mice and humans limit the interpretation and adaptation of the results. Marmoset monkey is a non-human primate (NHPs) with more similar reproductive physiology to humans. In this study, we attempted to establish a 3D matrix (Matrtigel)-based IVFM condition for marmoset ovarian follicles in combination with anti-apoptotic factor, X-linked inhibitor of apoptosis protein (XIAP).
METHODS:
Marmoset follicles were isolated as individual follicles and cultured in a single drop with the addition of 0, 10, and 100 μg/mL of XIAP molecules. Matured oocytes and granulosa cells from mature follicles were collected and analyzed. The average number of isolated follicles was less than 100, and primordial and antral follicles were abundant in the ovaries.
Results
IVFM of marmoset follicles in 3D matrix conditions with XIAP increased the rates of survival and in vitro follicle development. Furthermore, oocytes from the 3D cultures were successfully fertilized and developed in vitro. The addition of XIAP increased the secretion of estradiol and aromatase. Furthermore, expression of granulosa-specific genes, such as bone morphogenetic protein 15, Oct4, and follicle-stimulating hormone receptor were upregulated in the in vitro-matured follicles than in normal, well-grown, and atretic follicles. Apoptosis-related B-cell lymphoma-2 was highly expressed in the atretic follicles than in the XIAP-treated follicles, and higher caspase-3 was localized in the XIAP-treated follicles.
Conclusion
In this study, we attempted to establish a 3D-matrix-based marmoset IVFM condition and demonstrated the synergistic effects of XIAP. The use of a 3D matrix may be applied as an optimal culture condition for marmoset ovarian follicles.

The ovaries or female reproductive organs (FROs) are excised under anesthesia. Isolated ovaries are cut into pieces, and the follicles are isolated. All images are captured under a stereo microscope except for A. A excised FROs of marmoset monkeys; B isolated ovaries; C sliced half ovaries; D pieces of the ovaries and follicles within the ovarian tissue are imaged inside the circles

Histological observations of sectioned marmoset ovaries. The images of the follicles of each stage are enlarged in a blue-lined circle

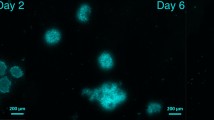
Isolated marmoset ovarian follicles are subjected to in vitro-maturation in a 2D or 3D environment with or without Xlinked inhibitor of apoptosis protein (XIAP). The in vitro development of the follicles is evaluated by changes in morphology with filled follicular fluid. Representative images of follicles are taken on days 3, -8, and -13. A in vitro follicular growth in 2D; B in vitro follicular growth in 3D

In vitro ovulation is induced by treatment with human chorionic gonadotropin (hCG), and movement to the follicle membrane region is observed. A ruptured oocyte is collected, and mature oocytes (MII) are further processed for in vitro fertilization (IVF). Fertilized eggs develop into the cleavage stage and into eight-cell stage in vitro. Representative images of A are taken on day 14. A Induction of ovulation by hCG treatment; B IVF of oocytes from in vitro follicular maturation

Secretion of estradiol (E2) and aromatase were evaluated by enzyme-linked immunosorbent assay. The specific expression of these genes was analyzed by quantitative reverse transcription-polymerase chain reaction. A concentration of E2 in in vitro matured marmoset ovarian follicles; B concentration of aromatase; C specific relative expression of granulosa cell-specific genes (p < 0.0001); D localization of follicle-stimulating hormone receptor (FSH Rc) in follicles grown in vitro

The expression of anti-apoptotic molecules is analyzed at mRNA and protein levels. A localization of caspase-3; B expression of Bcl-2
Similar content being viewed by others
References
Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9:735–49.
Abir R, Nitke S, Ben-Haroush A, Fisch B. In vitro maturation of human primordial ovarian follicles: clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21:887–98.
Herraiz S, Buigues A, Díaz-García C, Romeu M, Martínez S, Gómez-Seguí I, et al. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018;109:908–18.e2.
Demirtas E, Elizur SE, Holzer H, Gidoni Y, Son WY, Chian RC, et al. Immature oocyte retrieval in the luteal phase to preserve fertility in cancer patients. Reprod Biomed Online. 2008;17:520–3.
Kim YJ, Ku SY, Kim YY, Liu HC, Chi SW, Kim SH, et al. MicroRNAs transfected into granulosa cells may regulate oocyte meiotic competence during in vitro maturation of mouse follicles. Hum Reprod. 2013;28:3050–61.
Mostinckx L, Segers I, Belva F, Buyl R, Santos-Ribeiro S, Blockeel C, et al. Obstetric and neonatal outcome of ART in patients with polycystic ovary syndrome: IVM of oocytes versus controlled ovarian stimulation. Hum Reprod. 2019;34:1595–607.
Green LJ, Shikanov A. In vitro culture methods of preantral follicles. Theriogenology. 2016;86:229–38.
Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633–9.
Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–85.
Shikanov A, Xu M, Woodruff TK, Shea LD. A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3 dimensional system. J Vis Exp. 2011;49:2695.
Smith RM, Shikanov A, Kniazeva E, Ramadurai D, Woodruff TK, Shea LD. Fibrin-mediated delivery of an ovarian follicle pool in a mouse model of infertility. Tissue Eng Part A. 2014;20:3021–30.
Zhou H, Malik MA, Arab A, Hill MT, Shikanov A. Hydrogel based 3-Dimensional (3D) system for toxicity and high-throughput (HTP) analysis for cultured MURINE ovarian follicles. PLoS One. 2015;10:e0140205.
Kim YJ, Kim YY, Kang BC, Kim MS, Ko IK, Liu HC, et al. Induction of multiple ovulation via modulation of angiotensin II receptors in in vitro ovarian follicle culture models. J Tissue Eng Regen Med. 2017;11:3100–10.
Kim YJ, Park KE, Kim YY, Kim H, Ku SY, Suh CS, et al. Effects of estradiol on the paracrine regulator expression of in vitro maturated murine ovarian follicles. Tissue Eng Regen Med. 2017;14:31-8.
Park KE, Ku SY, Jung KC, Liu HC, Kim YY, Kim YJ, et al. Effects of urinary and recombinant gonadotropins on in vitro maturation outcomes of mouse preantral follicles. Reprod Sci. 2013;20:909–16.
Park KE, Kim YY, Ku SY, Baek SM, Huh Y, Kim YJ, et al. Effects of alginate hydrogels on in vitro maturation outcome of mouse preantral follicles. Tissue Eng Regen Med. 2012;9:170–4.
Rossetto R, Lima-Verde IB, Matos MH, Saraiva MV, Martins FS, Faustino LR, et al. Interaction between ascorbic acid and follicle-stimulating hormone maintains follicular viability after long-term in vitro culture of caprine preantral follicles. Domest Anim Endocrinol. 2009;37:112–23.
Murray AA, Molinek MD, Baker SJ, Kojima FN, Smith MF, Hillier SG, et al. Role of ascorbic acid in promoting follicle integrity and survival in intact mouse ovarian follicles in vitro. Reproduction. 2001;121:89–96.
Yun JW, Kim YY, Ahn JH, Kang BC, Ku SY. Use of nonhuman primates for the development of bioengineered female reproductive organs. Tissue Eng Regen Med. 2016;13:323–34.
Kim YY, Yun JW, Kim JM, Park CG, Rosenwaks Z, Liu HC, et al. Gonadotropin ratio affects the in vitro growth of rhesus ovarian preantral follicles. J Investig Med. 2016;64:888–93.
Gilchrist RB, Nayudu PL, Nowshari MA, Hodges JK. Meiotic competence of marmoset monkey oocytes is related to follicle size and oocyte-somatic cell associations. Biol Reprod. 1995;52:1234–43.
Kim YY, Kang BC, Yun JW, Ahn JH, Kim YJ, Kim H, et al. Expression of transcripts in marmoset oocytes retrieved during follicle isolation without gonadotropin induction. Int J Mol Sci. 2019;20:1133.
Konrad L, Kuhnert S, Nayudu PL, Einspanier R, Hinsch KD, Hinsch E. Quantification of ZP1, ZP2 and ZP3 mRNA of marmoset monkey (Callithrix jacchus) oocytes from periantral and antral follicles. Andrologia. 2012;44:349–53.
Galbán S, Duckett CS. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ. 2010;17:54–60.
Kim YY, Kim WO, Liu HC, Rosenwaks Z, Kim JW, Ku SY. Effects of paclitaxel and cisplatin on in vitro ovarian follicle development. Arch Med Sci. 2019;15:1510–9.
Kim YY, Tamadon A, Ku SY. Potential use of antiapoptotic proteins and noncoding RNAs for efficient in vitro follicular maturation and ovarian bioengineering. Tissue Eng Part B Rev. 2017;23:142–58.
Lee S, Ryu KJ, Kim B, Kang D, Kim YY, Kim T. Comparison between slow freezing and vitrification for human ovarian tissue cryopreservation and xenotransplantation. Int J Mol Sci. 2019;20:3346.
Kim YY, Kim JS, Che JH, Ku SY, Kang BC, Yun JW. Comparison of genetically engineered immunodeficient animal models for nonclinical testing of stem cell therapies. Pharmaceutics. 2021;13:130.
Mansfield K. Marmoset models commonly used in biomedical research. Comp Med. 2003;53:383-92.
Marmoset Genome S, Analysis C. The common marmoset genome provides insight into primate biology and evolution. Nat Genet. 2014;46:850–7.
Orsi A, Rees D, Andreini I, Venturella S, Cinelli S, Oberto G. Overview of the marmoset as a model in nonclinical development of pharmaceutical products. Regul Toxicol Pharmacol. 2011;59:19–27.
Grupen CG, Gilchrist RB, Nayudu PL, Barry MF, Schulz SJ, Ritter LJ, et al. Effects of ovarian stimulation, with and without human chorionic gonadotrophin, on oocyte meiotic and developmental competence in the marmoset monkey (Callithrix jacchus). Theriogenology. 2007;68:861–72.
Hanazawa K, Mueller T, Becker T, Heistermann M, Behr R, Sasaki E. Minimally invasive transabdominal collection of preimplantation embryos from the common marmoset monkey (Callithrix jacchus). Theriogenology. 2012;78:811–6.
Hirano T, Iwasaki YW, Lin ZY, Imamura M, Seki NM, Sasaki E, et al. Small RNA profiling and characterization of piRNA clusters in the adult testes of the common marmoset, a model primate. RNA. 2014;20:1223–37.
Kanda A, Nobukiyo A, Yoshioka M, Hatakeyama T, Sotomaru Y. Quality of common marmoset (Callithrix jacchus) oocytes collected after ovarian stimulation. Theriogenology. 2018;106:221–6.
Ogonuki N, Inoue H, Matoba S, Kurotaki YK, Kassai H, Abe Y, et al. Oocyte-activating capacity of fresh and frozen-thawed spermatids in the common marmoset (Callithrix jacchus). Mol Reprod Dev. 2018;85:376–86.
Tkachenko OY, Delimitreva S, Heistermann M, Scheerer-Bernhard JU, Wedi E, Nayudu PL. Critical estradiol dose optimization for oocyte in vitro maturation in the common marmoset. Theriogenology. 2015;83:1254–63.
Tkachenko OY, Delimitreva S, Isachenko E, Valle RR, Michelmann HW, Berenson A, et al. Epidermal growth factor effects on marmoset monkey (Callithrix jacchus) oocyte in vitro maturation, IVF and embryo development are altered by gonadotrophin concentration during oocyte maturation. Hum Reprod. 2010;25:2047–58.
Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials. 2011;32:2524–31.
Obexer P, Ausserlechner MJ. X-linked inhibitor of apoptosis protein - a critical death resistance regulator and therapeutic target for personalized cancer therapy. Front Oncol. 2014;4:197.
Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, et al. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999;18:179-87.
Phillipps HR, Hurst PR. XIAP: a potential determinant of ovarian follicular fate. Reproduction. 2012;144:165–76.
Funding
This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) (2019R1A2C1010163 and 2020R1A2C1010293).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the author(s).
Ethical statement
All animal experiments were approved by the IACUC of Seoul National University Hospital [SNUH IACUC No. 15–0032-C2A0(1)].
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, Y.Y., Yun, JW., Kim, S.W. et al. Synergistic Promoting Effects of X-Linked Inhibitor of Apoptosis Protein and Matrix on the In Vitro Follicular Maturation of Marmoset Follicles. Tissue Eng Regen Med 19, 93–103 (2022). https://doi.org/10.1007/s13770-021-00387-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00387-4