Abstract
Purpose
Differential diagnosis between acute viral and bacterial infection is an emerging common challenge for a physician in the emergency department. Serum C-reactive protein (CRP) is used to support diagnosis of bacterial infection, but in patients admitted with low CRP, its ability to discriminate between viral and bacterial infections is limited. We aimed to use two consecutive CRP measurements in order to improve differential diagnosis between bacterial and viral infection.
Methods
A single-center retrospective cohort (n = 1629) study of adult patients admitted to the emergency department with a subsequent microbiological confirmation of either viral or bacterial infection. Trend of CRP was defined as the absolute difference between the first two measurements of CRP divided by the time between them, and we investigated the ability of this parameter to differentiate between viral and bacterial infection.
Results
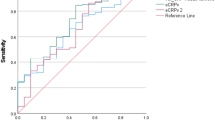
In patients with relatively low initial CRP concentration (< 60 mg/L, n = 634 patients), where the uncertainty regarding the type of infection is the highest, the trend improved diagnosis accuracy (AUC 0.83 compared to 0.57 for the first CRP measurement). Trend values above 3.47 mg/L/h discriminated bacterial from viral infection with 93.8% specificity and 50% sensitivity.
Conclusions
The proposed approach for using the kinetics of CRP in patients whose first CRP measurement is low can assist in differential diagnosis between acute bacterial and viral infection.




Similar content being viewed by others
References
Lindner HA, Thiel M, Schneider-Lindner V. Automated dynamic sepsis surveillance with routine data: opportunities and challenges. Ann Transl Med. 2017;5(3).
Rossolini GM, Arena F, Pecile P, Pollini S. Update on the antibiotic resistance crisis. Curr Opin Pharmacol. 2014;18:56–60.
Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761–3.
Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972–8.
van Houten CB, de Groot JA, Klein A, Srugo I, Chistyakov I, de Waal W, Meijssen CB, Avis W, Wolfs TF, Shachor-Meyouhas Y, Stein M. A host-protein based assay to differentiate between bacterial and viral infections in preschool children (OPPORTUNITY): a double-blind, multicentre, validation study. Lancet Infect Dis. 2017;17:431–40.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10.
ten Oever J, Netea MG, Kullberg BJ. Utility of immune response-derived biomarkers in the differential diagnosis of inflammatory disorders. J Infect. 2016;72:1–8.
Rainer TH, Chan CP, Leung MF, Leung W, Ip M, Lee N, Cautherley GW, Graham CA, Fuchs D, Renneberg R. Diagnostic utility of CRP to neopterin ratio in patients with acute respiratory tract infections. J Infect. 2009;58:123–30.
Ip M, Rainer TH, Lee N, Chan C, Chau SS, Leung W, Leung MF, Tam TK, Antonio GE, Lui G, Lau TK. Value of serum procalcitonin, neopterin, and C-reactive protein in differentiating bacterial from viral etiologies in patients presenting with lower respiratory tract infections. Diagn Microbiol Infect Dis. 2007;59:131–6.
Chan YL, Liao HC, Tsay PK, Chang SS, Chen JC, Liaw SJ. C-reactive protein as an indicator of bacterial infection of adult patients in the emergency department. Chang Gung Med J. 2002;25:437–45.
Kofoed K, Andersen O, Kronborg G, Tvede M, Petersen J, Eugen-Olsen J, Larsen K. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care. 2007;11:R38.
Nuutila J, Jalava-Karvinen P, Hohenthal U, Kotilainen P, Pelliniemi TT, Nikoskelainen J, Lilius EM. A rapid flow cytometric method for distinguishing between febrile bacterial and viral infections. J Microbiol Methods. 2013;92:64–72.
Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, Ortqvist A, Schaberg T, Torres A, Van Der Heijden G, Read R. Guidelines for the management of adult lower respiratory tract infections-full version. Clin Microbiol Infect. 2011;17:E1–59.
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54.
Pfister R, Kochanek M, Leygeber T, Brun-Buisson C, Cuquemelle E, Machado MP, Piacentini E, Hammond NE, Ingram PR, Michels G. Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: a prospective cohort study, systematic review and individual patient data meta-analysis. Crit Care. 2014;18:R44.
Wasserman A, Karov R, Shenhar-Tsarfaty S, Paran Y, Zeltzer D, Shapira I, Trotzky D, Halpern P, Meilik A, Raykhshtat E, Goldiner I. Septic patients presenting with apparently normal C-reactive protein: a point of caution for the ER physician. Medicine 2019;98(2).
Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. 2013;13:1057–98.
Ziv-Baran T, Wasserman A, Shteinvil R, Zeltser D, Shapira I, Shenhar-Tsarfaty S, Meilik A, Goldiner I, Rogowski O, Berliner S, Halpern P. C-reactive protein and emergency department seven days revisit. Clin Chim Acta. 2018;481:207–11.
Póvoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, Sabino H. Pilot study evaluating C-reactive protein levels in the assessment of response to treatment of severe bloodstream infection. Clin Infect Dis. 2005;40:1855–7.
Povoa P, Teixeira-Pinto AM, Carneiro AH. Portuguese Community-Acquired Sepsis Study Group S: C-reactive protein, an early marker of community-acquired sepsis resolution: a multi-center prospective observational study. Crit Care. 2011;15:R169.
Schmit X, Vincent JL. The time course of blood C-reactive protein concentrations in relation to the response to initial antimicrobial therapy in patients with sepsis. Infection. 2008;36:213–9.
Cooke CR, Iwashyna TJ. Using existing data to address important clinical questions in critical care. Critical Care Med. 2013;41:886–96.
Sasaki K, Fujita I, Hamasaki Y, Miyazaki S. Differentiating between bacterial and viral infection by measuring both C-reactive protein and 2′-5′-oligoadenylate synthetase as inflammatory markers. J Infect Chemother. 2002;8:76–80.
Lobo SM. Sequential C-reactive protein measurements in patients with serious infections: does it help? Crit Care. 2012;16:130.
Coelho LM, Salluh JI, Soares M, Bozza FA, Verdeal JR, Castro-Faria-Neto HC, e Silva JR, Bozza PT, Póvoa P. Patterns of C-reactive protein RATIO response in severe community-acquired pneumonia: a cohort study. Crit Care. 2012;16:R53.
Hoeboer SH, Groeneveld AJ, Engels N, van Genderen M, Wijnhoven BP, van Bommel J. Rising C-reactive protein and procalcitonin levels precede early complications after esophagectomy. J Gastrointest Surg. 2015;19:613–24.
Beloosesky Y, Grinblat J, Pirotsky A, Weiss A, Hendel D. Different C-reactive protein kinetics in post-operative hip-fractured geriatric patients with and without complications. Gerontology. 2004;50:216–22.
Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–58.
Goldshtein I, Neeman U, Chodick G, Shalev V. Variations in hemoglobin before colorectal cancer diagnosis. Eur J Cancer Prev. 2010;19:342–4.
Paran Y, Yablecovitch D, Choshen G, Zeitlin I, Rogowski O, Ben-Ami R, Katzir M, Saranga H, Rosenzweig T, Justo D, Orbach Y. C-reactive protein velocity to distinguish febrile bacterial infections from non-bacterial febrile illnesses in the emergency department. Crit Care. 2009;13:R50.
Nargis W, Ibrahim MD, Ahamed BU. Procalcitonin versus C-reactive protein: usefulness as biomarker of sepsis in ICU patient. Int J Crit Illn Injury Sci. 2014;4:195.
ten Oever J, Tromp M, Bleeker-Rovers CP, Joosten LA, Netea MG, Pickkers P, van de Veerdonk FL. Combination of biomarkers for the discrimination between bacterial and viral lower respiratory tract infections. J Infect. 2012;65:490–5.
Menéndez R, Sahuquillo-Arce JM, Reyes S, Martínez R, Polverino E, Cillóniz C, Córdoba JG, Montull B, Torres A. Cytokine activation patterns and biomarkers are influenced by microorganisms in community-acquired pneumonia. Chest. 2012;141:1537–45.
Krüger S, Ewig S, Papassotiriou J, Kunde J, Marre R, von Baum H, Suttor N, Welte T, CAPNETZ Study Group. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP—results from the German competence network CAPNETZ. Respir Res. 2009;10:65.
Acknowledgements
R.S. was supported in part by the Israel Science Foundation (Grant 1339/18), by Grant 2016694 from the United States–Israel Binational Science Foundation (BSF), Jerusalem, Israel, and the United States National Science Foundation (NSF), and by the Naomi Prawer Kadar Foundation. S.S.T. was supported by the ELROV grant. D.C. was supported, in part, by fellowships from the Edmond J. Safra Center for Bioinformatics at Tel Aviv University and from Google.
Funding
There is no funding to report for this submission.
Author information
Authors and Affiliations
Contributions
DC—planned and conducted the study, collected and interpreted data, drafted the manuscript and approved the final submission. AW—collected and interpreted data, drafted the manuscript and approved the final submission. EF—collected and interpreted data, drafted the manuscript and approved the final submission. OR—planned and conducted the study, drafted the manuscript and approved the final submission. DZ—collected data and approved the final submission. IS—collected data and approved the final submission. DB—collected and interpreted data, drafted the manuscript and approved the final submission. AM—collected data and approved the final submission. ER—collected data and approved the final submission. PH—collected data and approved the final submission. SB—planned the study, interpreted data and approved the final submission. SS-T—planned the study, interpreted data, drafted the manuscript and approved the final submission. RS—planned and supervised the study, interpreted data, drafted the manuscript and approved the final submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Ethics approval
The study was reviewed and approved by Sourasky Tel Aviv Medical Center Institutional Review Board (number 0491-17).
Consent for publication
The work described has not been published before and it is not under consideration for publication anywhere else. This publication has been approved by all co-authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coster, D., Wasserman, A., Fisher, E. et al. Using the kinetics of C-reactive protein response to improve the differential diagnosis between acute bacterial and viral infections. Infection 48, 241–248 (2020). https://doi.org/10.1007/s15010-019-01383-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-019-01383-6