Abstract
Purpose
Current German/Austrian antiretroviral treatment guidelines recommend more than 20 combination regimens for first-line therapy, without a preference. Regimens include two nucleoside reverse transcriptase inhibitors (NRTIs) plus either an integrase strand transfer inhibitor (INSTI), a non-NRTI (NNRTI) or a boosted protease inhibitor (PI). The objective was to examine the outcomes of recommended first-line ART in Germany.
Methods
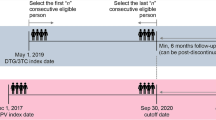
This nationwide observational study included treatment-naïve chronically HIV-1 infected patients receiving one of the recommended first-line regimens. Patients were allocated to three arms (INSTI, NNRTI, PI) and were prospectively followed for 24 months. Delayed treatment initiation was defined by a baseline CD4 T-cell count of < 350/µl or CDC clinical stage C.
Results
Among a total of 434 patients enrolled, virologic failure was rare and occurred in 4.3% (6/141) in the PI arm, in 3.3% (4/122) in the NNRTI arm and in 0.6% (1/171) in the INSTI arm (p = 0.10). De novo drug resistance mutations developed in only two patients in the NNRTI arm. Nonetheless, treatment modifications were frequent (51%) and mostly performed for strategic reasons. Retention on all initial compounds at month 24 was 64%, 49%, and 22% in the INSTI, NNRTI and PI arms respectively. Delayed treatment initiation was common (47%) and more frequently observed in patients in the PI arm. It was not associated with virological failure.
Conclusion
High efficacy and low virological failure rates were observed with recommended first-line regimens independent of delayed treatment initiation, chosen regimen and subsequent treatment modifications, demonstrating the validity of the current treatment guidelines.


Similar content being viewed by others
References
Deutsche AIDS-Gesellschaft, Österreichische AIDS-Gesellschaft. Deutsch-Österreichische Leitlinien zur antiretroviralen Therapie der HIV-Infektion. https://daignet.de/site-content/hiv-leitlinien/hiv-leitlinien. Accessed Nov 2019.
EACS. European Guidelines for treatment of HIV-positive adults in Europe 10.0. https://www.eacsociety.org/files. Accessed Nov 2019.
Carr A, Richardson R, Liu Z. Success and failure of initial antiretroviral therapy in adults: an updated systematic review. AIDS. 2019;33:443–53.
Schäfer G, Kreuels B, Schmiedel S, Hertling S, Hüfner A, Degen O, van Lunzen J, Schulze Z, Wiesch J. High proportion of HIV late presenters at an academic tertiary care center in northern Germany confirms the results of several cohorts in Germany: time to put better HIV screening efforts on the national agenda? Infection. 2016;44:347–52.
Stecher M, Schommers P, Schmidt D, Kollan C, Gunsenheimer-Bartmeyer B, Lehmann C, Platten M, Fätkenheuer G, Vehreschild JJ, ClinSurv Study Group. Antiretroviral treatment indications and adherence to the German-Austrian treatment initiation guidelines in the German ClinSurv HIV Cohort between 1999 and 2016. Infection. 2019;47:247–55.
Eaton EF, Tamhane A, Davy-Mendez T, et al. Trends in antiretroviral therapy prescription, durability and modification: new drugs, more changes, but less failure. AIDS. 2018;32:347–55.
Davy-Mendez T, Eron JJ, Zakharova O, Wohl DA, Napravnik S. Increased persistence of initial treatment for hiv infection with modern antiretroviral therapy. J Acquir Immune Defic Syndr. 2017;76:111–5.
d’Arminio Monforte A, Lorenzini P, Cozzi-Lepri A, et al. Durability and tolerability of first-line regimens including two nucleoside reverse transcriptase inhibitors and raltegravir or ritonavir boosted-atazanavir or -darunavir: data from the ICONA Cohort. HIV Clinical Trials. 2018;19:52–60.
Lathouwers E, Wong EY, Luo D, Seyedkazemi S, De Meyer S, Brown K. HIV-1 resistance rarely observed in patients using darunavir once-daily regimens across clinical studies. HIV Clin Trials. 2017;18:196–204.
El Bouzidi K, White E, Mbisa JL, et al. HIV-1 drug resistance mutations emerging on darunavir therapy in PI naive and -experienced patients in the UK. J Antimicrob Chemother. 2016;71:3487–94.
Santos JR, Cozzi-Lepri A, Phillips A, et al. Long-term effectiveness of recommended boosted protease inhibitor-based antiretroviral therapy in Europe. HIV Med. 2018;19:324–38.
Zeder AJ, Hilge R, Schrader S, Bogner JR, Seybold U. Medium-grade tubular proteinuria is common in HIV-positive patients and specifically associated with exposure to tenofovir disoproxil Fumarate. Infection. 2016;44:641–9.
Young J, Smith C, Teira R, et al. Antiretroviral pill count and clinical outcomes in treatment-naïve patients with HIV infection. HIV Med. 2018;19:132–42.
Griffith DC, Farmer C, Gebo KA, et al. Uptake and virological outcomes of single-versus multi-tablet antiretroviral regimens among treatment-naïve youth in the HIV Research Network. HIV Med. 2019;20:169–74.
Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence. 2019;13:475–90.
Li SL, Xu P, Zhang L, Sun GX, Lu ZJ. Effectiveness and safety of rilpivirine, a non-nucleoside reverse transcriptase inhibitor, in treatment-naive adults infected with HIV-1: a meta-analysis. HIV Clin Trials. 2014;15:261–8.
Llibre JM, Pulido F, García F, García Deltoro M, Blanco JL. Delgado R Genetic barrier to resistance for dolutegravir. AIDS Rev. 2015;17:56–64.
d'Arminio Monforte A, Cozzi-Lepri A, Di Biagio A, et al. Durability of first-line regimens including integrase strand transfer inhibitors (INSTIs): data from a real-life setting. J Antimicrob Chemother. 2019;74:1363–7.
Nance RM, Vannappagari V, Smith K, et al. Virologic failure among people living with HIV initiating dolutegravir-based versus other recommended regimens in real-world clinical care settings. J Acquir Immune Defic Syndr. 2019;81:572–7.
Zoufaly A, Kraft C, Schmidbauer C, Puchhammer-Stoeckl E. Prevalence of integrase inhibitor resistance mutations in Austrian patients recently diagnosed with HIV from 2008 to 2013. Infection. 2017;45:165–70.
Engler K, Lessard D, Lebouché B. A review of HIV-specific patient-reported outcome measures. Patient. 2017;10:187–202.
Lifson AR, Grund B, Gardner EM, et al. Improved quality of life with immediate versus deferred initiation of antiretroviral therapy in early asymptomatic HIV infection. AIDS. 2017;31:953–63.
Acknowledgements
We thank Nikola Hanhoff from dagnae e.V., Berlin, and Birgit Mück from MUC Research for study coordination and monitoring, Paul Lauscher from MUC Research, Munich, for statistical analyses. We thank all participating patients as well as the staff and investigators of the PROPHET study group: Practice Kreuzberg, Hubert Schulbin, Berlin; Center for Infectiology Berlin Prenzlauer Berg GmbH (Zibp), Axel Baumgarten, Christoph Mayr, Berlin; MVZ Aerzteforum Seestrasse, Wolfgang Schmidt, Berlin; Department of Medicine I, Bonn University Hospital, Jürgen Rockstroh, Bonn; Praxis Ebertplatz, Christoph Wyen, Esther Voigt, Tim Kümmerle, Cologne; Practice Hohenstaufenring, Stefan Scholten, Stephan Schneeweiss, Cologne; Department I of Internal Medicine, University Hospital of Cologne, Gerd Fätkenheuer, Cologne; Center for HIV and Hepatogastroenterology, Stefan Mauss, Düsseldorf; University HIV/STD Center, Essen, Department of Dermatology and Venerology, University Hospital Essen, Stefan Esser, Essen; Infektiologikum Frankfurt, Markus Bickel, Stephan Klauke, Thomas Lutz; Infektiologikum Freiburg Susanne Usadel; Practice Dr. Frank Ackermann, Halle; ifi-Studien und Projekte GmbH an der Asklepios Klinik St. Georg, Haus L, Albrecht Stoehr, Andreas Plettenberg, Hamburg; ICH Studycenter, Christian Hoffmann, Hans-Jürgen Stellbrink, Michael Sabranski, Carl Knud Schewe, Hamburg; Hannover Medical School, Matthias Stoll, Hannover; Center for Medical Studies, Hans Heiken, Hannover; MVZ Karlsplatz, HIV Research and Clinical Care Centre, Munich, Hans Jäger, Munich; Practice Isartor, Ramona Pauli, Werner Becker, Munich; University Hospital of Munich, Johannes Bogner, Munich; Technical University of Munich, School of Medicine, Christoph D. Spinner, Munich; prinzmed, Practice for infectious diseases, Nils Postel, Munich; Center for Interdisciplinary Medicine, Stefan Christensen, Muenster; Practice Schwabstrasse 26, Markus Mueller, Albrecht Ulmer, Stuttgart; Institute for Health Care Management and Research, Jürgen Wasem, Anja Neumann, Duisburg-Essen.
Funding
We thank Janssen-Cilag for providing the financial support for the conduct of study.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Eva Wolf and Stefan Preis. The first draft of the manuscript was written by Markus Bickel and Christian Hoffmann and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Markus Bickel has received honoraria for lecturing from the Hess.Arbeitsgemeinschaft der HIV-Versorger (HIVAG), travelling grants from Deutsche Arbeitsgemeinschaft niedergelassener Ärzte in der Versorgung HIV-Infizierter e. V. (dagnä) and has received research grants from AbbVie, Janssen-Cilag, MSD, Gilead Sciences, Glaxo Smith Kline, dagnä, Deutsche Leberstiftung, Institut für HIV Forschung Universitätsklinikum Essen, Infektionsmedizinisches Zentrum Hamburg (ICH). Christian Hoffmann has received honoraria for lecturing, advisory boards and/or travelling grants from AbbVie, EusaPharm, Gilead Sciences, HEXAL, Hormosan, Janssen-Cilag, MSD and ViiV Healthcare; he has also received research grants from AbbVie, Janssen-Cilag, MSD and Gilead Sciences. Stephan Klauke has received travelling grants from Deutsche Arbeitsgemeinschaft niedergelassener Ärzte in der Versorgung HIV-Infizierter e. V. (dagnä) and has received research grants from AbbVie, Janssen-Cilag, MSD, Gilead Sciences, Glaxo Smith Kline, dagnä, Deutsche Leberstiftung, Institut für HIV Forschung Universitätsklinikum Essen, Infektionsmedizinisches Zentrum Hamburg (ICH). Christoph D. Spinner has received grants for travel and participation in advisory boards or speaker’s honoraria from AbbVie, Astellas, Bristol-Meyers Squibb, Gilead, Janssen-Cilag, MSD, and ViiV Healthcare. He has also received grants for investigator-sponsored studies from Gilead Sciences, Janssen-Cilag, and ViiV Healthcare. Eva Wolf has received grants for travel and participation in advisory boards or speaker’s honoraria from AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, MSD Sharp & Dohme, Roche, and ViiV Healthcare. Knud Schewe has received speakers honoraria as well as honoraria for advisory boards and/or travel grants from AbbVie, Gilead Sciences, HEXAL, Janssen-Cilag, MSD and ViiV Healthcare as well as travel grants and conference sponsoring from Deutsche Arbeitsgemeinschaft niedergelassener Ärzte in der Versorgung HIV-Infizierter e. V. (dagnä). Axel Baumgarten has received honoraria for lecturing, advisory boards and/or travelling grants from AbbVie, Gilead Sciences, Janssen, Sanofi, MSD and ViiV Healthcare. Stefan Esser has received honoraria for lecturing, advisory boards and/or travelling grants from AbbVie, Gilead Sciences, HEXAL, Janssen-Cilag, MSD and ViiV Healthcare; he has also received research grants from ViiV, Janssen-Cilag, MSD and Gilead Sciences. Christoph Wyen has received grants for travel and participation in advisory boards or speaker’s honoraria from AbbVie, Bristol-Meyers Squibb, Gilead, Janssen-Cilag, MSD, and ViiV Healthcare. On behalf of all other authors, the corresponding author states that there is no conflict of interest.
Ethics approval
The primary ethical approval was received by the University Duisburg/Essen on June 25, 2014 (ID 14-5847-BO), the study was registered at the Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, ID7237), the National Association of Statutory Health Insurance Funds (GKV Spitzenverband, ID40128) and the German clinical trial registry (Deutsche Register Klinischer Studien, ID 00006321).
Consent to participate
Partcipating centers signed a contract for the conduction of the study with the Deutsche Arbeitsgemeinschaft niedergelassener Ärzte in der Versorgung HIV-Infizierter e. V. (dagnä) e. V. located in Berlin.
Consent for publication
All co-authors have read the manuscript had sufficient time to consider changes and finally agreed to its publication in the present form.
Availability of data and material
The data of the study were presented and discussed at all investigator meetings which were held twice yearly over the entire time the study was conducted in conjunction with two national HIV meetings.
Code availability
Statistical analysis was conducted using Stata/SE 15.1 (StataCorp LLC, Texas, USA).
Additional information
The members of the investigators of the PROPHET study group are listed in acknowledgements.
Rights and permissions
About this article
Cite this article
Bickel, M., Hoffmann, C., Wolf, E. et al. High effectiveness of recommended first-line antiretroviral therapies in Germany: a nationwide, prospective cohort study. Infection 48, 453–461 (2020). https://doi.org/10.1007/s15010-020-01428-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-020-01428-1