Abstract
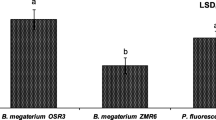
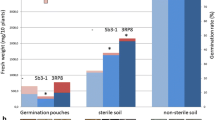
Bacterial wilt caused by the vascular pathogen Ralstonia solanacearum is a major constraint for cultivation of eggplant (Solanum melongena L.) in the state of Goa, India. Three strains of endophytic bacteria isolated from xylem sap of eggplant and antagonistic to R. solanacearum were evaluated for colonization of eggplant cv. Agassaim under greenhouse conditions. Results suggest that rifampicin resistant mutants XB1R (Agrobacterium tumefaciens), XB99R (Enterobacter sp.) and XB177R (Bacillus cereus) exhibit varying degree of colonization of endophytic tissues and rhizosphere soil of eggplant. XB177R colonized eggplant most efficiently and systemically from rhizosphere (6.5 Log cfu g−1) to leaves (4.34 Log cfu g−1) until 30 days after inoculation. Green fluorescent protein tagged XB177 colonized rhizoplane, cortex and xylem vessels of eggplant grown under greenhouse conditions. Studies on changes in the population of XB177R during bacterial wilt infection suggested that its endophytic population of 5.48 log cfu g−1 fresh weight and rhizosphere population of 6.18 log cfu g−1 of rhizosphere soil was necessary to control bacterial wilt in eggplant. Further, it is reported that pre-treatment of eggplant is an important pre-requisite for effective wilt prevention by the antagonistic bacteria. The present study demonstrates the importance of plant colonization ability of antagonistic bacteria for bacterial wilt control.



Similar content being viewed by others
References
Reinhold-Hurek B, Hurek T (2011) Living inside plants: bacterial endophytes. Curr Opin Plant Biol 14:435–443
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37:634–663
Tan S, Jiang Y, Song S, Huang J, Ling N, Xu Y, Shen Q (2013) Two Bacillus amyloliquefaciens strains isolated using the competitive tomato root enrichment method and their effects on suppressing R. solanacearum and promoting tomato plant growth. Crop Prot 43:134–140. https://doi.org/10.1016/j.cropro.2012.08.003
Achari GA, Ramesh R (2014) Diversity, biocontrol, and plant growth promoting abilities of xylem residing bacteria from solanaceous crops. Int J Microbiol. https://doi.org/10.1155/2014/296521
Götz M, Gomes NCM, Dratwinski A, Costa R, Berg G, Peixoto R, Mendonca-Hagler L, Smalla K (2006) Survival of gfp-tagged antagonistic bacteria in the rhizosphere of tomato plants and their effects on the indigenous bacterial community. FEMS Microbiol Ecol 56:207–218
Ji X, Lu G, Gai Y, Zheng C, Mu Z (2008) Biological control against bacterial wilt and colonization of mulberry by an endophytic Bacillus subtilis strain. FEMS Microbiol Ecol 65:565–573
Ghirardi S, Dessaint F, Mazurier S, Corberand T, Raaijmakers JM, Meyer J-M, Dessaux Y, Lemanceau P (2012) Identification of traits shared by rhizosphere-competent strains of fluorescent Pseudomonads. Microb Ecol 64:725–737
Ji X, Lu G, Gai Y, Gao H, Lu B, Kong L, Mu Z (2010) Colonization of Morus alba L. by the plant growth-promoting and antagonistic bacterium Burkholderia cepacia strain Lu10-1. BMC Microbiol 10:243. http://www.biomedcentral.com/1471-2180/10/243
Ramesh R, Achari GA, Gaitonde S (2014) Genetic diversity of Ralstonia solanacearum infecting solanaceous vegetables from India reveals the existence of unknown or newer sequevars of Phylotype I strains. Eur J Plant Pathol 140:543–562. https://doi.org/10.1007/s10658-014-0487-5
Achari GA, Ramesh R (2015) Characterization of bacteria degrading 3-hydroxy palmitic acid methyl ester (3OH-PAME), a quorum sensing molecule of Ralstonia solanacearum. Lett Appl Microbiol 60(5):447–455. https://doi.org/10.1111/lam.12389
Versalovic J, Scheider M, de Bruijn FJ, Lupski JR (1994) Genomic fingerprinting of bacteria using repetitive sequence based polymerase chain reaction. Method Mol Cell Biol 5:25–40
Ramesh R, Phadke GS (2012) Rhizosphere and endophytic bacteria for the suppression of eggplant wilt caused by Ralstonia solanacearum. Crop Prot 37:35–41
Buyer SJ (1995) A soil and rhizosphere microorganism isolation and enumeration medium that inhibits Bacillus mycoides. Appl Environ Microbiol 61(5):1839–1842
SAS Institute (2012) SAS/STAT User’s Guide, Version 9.2, 4(1). SAS Institute, Cary
Dunn AK, Handelsman J (1999) A vector for promoter trapping in Bacillus cereus. Gene 226:297–305
Dunn AK, Klimocwicz AK, Handelsman J (2003) Use of a promoter trap to identify Bacillus cereus genes regulated by tomato seed exudate and a rhizosphere resident, Pseudomonas aureofaciens. Appl Environ Microbiol 69(2):1197–1205
Ramos HJO, Roncato-Maccari LDB, Souza EM, Soares-Ramos JRL, Hungria M, Pedrosa FO (2002) Monitoring Azospirillum-wheat interactions using the gfp and gusA genes constitutively expressed from a new broad-host range vector. J Biotechnol 97:243–252
Germaine K, Keogh E, Garcia-Cabellos G, Borremans B, van der Lelie D, Barac T, Oeyen L, Vangronsveld J, Porteous Moore F, Moore ERB, Campbell CD, Ryan D, Dowling DN (2004) Colonisation of poplar trees by GFP expressing bacterial endophytes. FEMS Microbiol Ecol 48:109–118
Compant S, Reiter B, Sessitsch A, Nowak J, Clement C, Barka EA (2005) Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl Environ Microbiol 71(4):1685–1693
Verma SC, Ladha JK, Tripathi AK (2001) Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol 91:127–141
Saleh OI, Huang PY, Huang JS (1997) Bacillus pumilus, the cause of bacterial blotch of immature balady peach in Egypt. J Phytopathol 145(10):447–453
Timmusk S, Grantcharova N, Wagner EG (2005) Paenibacillus polymyxa invades plant roots and forms biofilms. Appl Environ Microbiol 71:7292–7300
Taghavi S, van der Lelie D, Hoffman A, Zhang Y-B, Walla MD et al (2010) Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet 6(5):e1000943. https://doi.org/10.1371/journal.pgen.1000943
Buschart A, Sachs S, Chen X, Herglotz J, Krause A, Reinhold-Hurek B (2012) Flagella mediate endophytic competence rather than act as MAMPS in Rice-Azoarcus sp. strain BH72 interactions. Mol Plant Microbe Interact 25(2):191–199
Melnick RL, Zidack NK, Bailey BA, Maximova SN, Guiltinan M, Backman PA (2008) Bacterial endophytes: Bacillus spp. from annual crops as potential biological control agents of black pod rot of cacao. Biol Control 46:46–56
Tkalec M, Car D, Gospocic J, Kirziac I, Duz K, Vidakovic-Cifrek K (2012) Response of Kalanchoe daigremontiana to wounding and infection with Agrobacterium tumefaciens. Period Biol 114(1):83–90
Anuratha CS, Gnanamanickam SS (1990) Biological control of bacterial wilt caused by Pseudomonas solanacearum in India with antagonistic bacteria. Plant Soil 124(1):109–116
Santiago TR, Grabowski C, Rossato M, Romeiro RS, Mizubuti ES (2015) Biological control of eucalyptus bacterial wilt with rhizobacteria. Biol Control 80:14–22
Rahman MM, Ali ME, Khan AA, Akanda AM, Uddin MdK, Hashim U, Abd Hamid SB (2012) Isolation, characterization, and Identification of biological control agent for potato soft rot in Bangladesh. Sci World J. https://doi.org/10.1100/2012/723293
Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R, Guo JH (2013) Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol 15(3):848–864
Acknowledgements
Financial support from Indian Council of Agricultural Research (ICAR), New Delhi, through “Outreach project on Phytophthora, Fusarium and Ralstonia diseases of horticultural and field crops”-(PhytoFuRa) and the facilities provided by the Director, ICAR-Central Coastal Agricultural Research Institute (formerly known as ICAR Research Complex for Goa), Goa, India, are greatly acknowledged. The authors are thankful to Dr. Daniel Zeigler, Bacillus Genetic Stock Centre, Ohio State University, Columbus, USA for providing the GFP vector pAD43-25, Prof. Jo Handelsman, Yale University, Connecticut, USA for E. coli strain GM2929 and Prof. S. K. Dubey, Department of Microbiology, Goa University for the E-porator facility.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest to publish this manuscript.
Additional information
Significance statement Bacterial wilt caused by Ralstonia solanacearum is a major constraint for cultivation of eggplant in India. Study on colonization of eggplant by antagonistic endophytic bacteria reveals successful colonization of bacteria in eggplant and pre-treatment of eggplant with antagonistic bacteria is an important pre-requisite for effective suppression of bacterial wilt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Achari, G.A., Ramesh, R. Colonization of Eggplant by Endophytic Bacteria Antagonistic to Ralstonia solanacearum, the Bacterial Wilt Pathogen. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 585–593 (2019). https://doi.org/10.1007/s40011-018-0972-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-018-0972-2