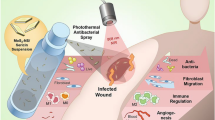
Abstract
There is a growing demand of bioinspired wound-healing platforms with superior tissue repairing and low immunogenicity. Hyaluronic acid (HA) and sericin (Sc) are extensively used for their competence in tissue engineering and gene/drug delivery. Herein, we demonstrate the formation of hybrid nanoparticles of HA and Sc (HS NPs) taking advantage of the dynamic disulfide bonding between their thiolated derivatives. The fabricated HS NPs were loaded with ursodeoxycholic acid (UDC), a known suppressor for NF-κB expression, and explored for the cellular response of designed hybrid UDC-HS NPs. The cytotoxicity of the synthesized hybrid system was evaluated on CCD-986Sk cells, showing negligible cytotoxicity with the cell proliferation data supporting the potential of UDC-HS NPs in increasing the cell density. Moreover, UDC-HS NPs also directed enhanced antioxidant activity. Furthermore, the wound-healing capability of UDC-HS NPs was investigated through in vitro scratch assay on CCD-986Sk cells. The results obtained could provide a useful approach in designing hybrid nanoplatform with implications in wound repair and tissue regeneration.









Similar content being viewed by others
Data availability
The data that support the tables within this article and other findings of this study are available from the corresponding author upon reasonable request.
References
Qi, C., Xu, L., Deng, Y., Wang, G., Wang, Z., Wang, L.: Sericin hydrogels promote skin wound healing with effective regeneration of hair follicles and sebaceous glands after complete loss of epidermis and dermis. Biomater. Sci. 6, 2859–2870 (2018)
Dreifke, M.B., Jayasuriya, A.A., Jayasuriya, A.C.: Current wound healing procedures and potential care. Mater. Sci. Eng. C 48, 651–662 (2015)
Lim, H.W., Collins, S.A.B., Resneck, J.S., Jr., Bolognia, J., Hodge, J.A., Rohrer, T.A., Van Beek, M.J., Margolis, D.J., Sober, A.J., Weinstock, M.A., Nerenz, D.R., Begolka, W.S., Moyano, J.V.: A risk adjustment approach to estimating the burden of skin disease in the united states. J. Am. Acad. Dermatol. 78, 129–140 (2018)
Li, Y., Liu, X., Tan, L., Cui, Z., Yang, X., Zheng, Y., Yeung, K.W.K., Chu, P.K., Wu, S.: Rapid sterilization and accelerated wound healing using Zn2+ and graphene oxide modified g-C3N4 under dual light irradiation. Adv. Funct. Mater. 28, 1800299 (2018)
Dhand, C., Venkatesh, M., Barathi, V.A., Harini, S., Bairagi, S., Goh-Tze-Leng, E., Muruganandham, N., Low, K.Z.W., Fazil, M., Loh, X.J., Srinivasan, D.K., Liu, S.P., Beuerman, R.W., Verma, N.K., Ramakrishna, S., Lakshminarayanan, R.: Bio-inspired crosslinking and matrix-drug interactions for advanced wound dressings with long-term antimicrobial activity. Biomaterials 138, 153–168 (2017)
Raaymakers, C., Verbrugghe, E., Hernot, S., Hellebuyck, T., Betti, C., Peleman, C., Claeys, M., Bert, W., Caveliers, V., Ballet, S., Martel, A., Pasmans, F., Roelants, K.: Antimicrobial peptides in frog poisons constitute a molecular toxin delivery system against predators. Nat. Commun. 8, 1495 (2017)
Ramanathan, G., Thyagarajan, S., Sivagnanam, U.T.: Accelerated wound healing and its promoting effects of biomimetic collagen matrices with siderophore loaded gelatin microspheres in tissue engineering. Mater. Sci. Eng. C 93, 455–464 (2018)
Mantri, Y., Tsujimoto, J., Donovan, B., Fernandes, C.C., Garimella, P.S., Penny, W.F., Anderson, C.A., Jokerst, J.V.: Photoacoustic monitoring of angiogenesis predicts response to therapy in healing wounds. Wound Repair Regen. 30, 258–267 (2022)
Vågesjö, E., Öhnstedt, E., Mortier, A., Lofton, H., Huss, F., Proost, P., Roos, S., Phillipson, M.: Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc. Natl. Acad. Sci. 115, 1895–1900 (2018)
Jin, L., Guo, X., Gao, D., Wu, C., Hu, B., Tan, G., Du, N., Cai, X., Yang, Z., Zhang, X.: Nir-responsive mxene nanobelts for wound healing. NPG Asia Mater. 13, 24 (2021)
Lu, X., Qin, L., Guo, M., Geng, J., Dong, S., Wang, K., Xu, H., Qu, C., Mia, J., Liu, M.: A novel alginate from Sargassum seaweed promotes diabetic wound healing by regulating oxidative stress and angiogenesis. Carbohyd. Polym. 289, 119437 (2022)
Goh, E.T., Kirby, G., Jayakumar, R., Liang, X.J., Tan, A.: Accelerated wound healing using nanoparticles, pp. 287–306. Academic Press, Boston (2016).. (M.R. Hamblin, P. Avci, T. W. Prow (eds))
Makabenta, J.M.V., Nabawy, A., Li, C.-H., Schmidt-Malan, S., Patel, R., Rotello, V.M.: Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 19, 23–36 (2021)
Skóra, B., Krajewska, U., Nowak, A., Dziedzic, A., Barylyak, A., Kus-Liśkiewicz, M.: Noncytotoxic silver nanoparticles as a new antimicrobial strategy. Sci. Rep. 11, 13451 (2021)
Liang, Y., He, J., Guo, B.: Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 15, 12687–12722 (2021)
Guo, B., Dong, R., Liang, Y., Li, M.: Haemostatic materials for wound healing applications. Nat. Rev. Chem. 5, 773–791 (2021)
Liang, Y., Zhao, X., Hu, T., Chen, B., Yin, Z., Ma, X.P., Guo, B.: Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 15, 1900046 (2019)
Hasanin, M., Taha, N.F., Abdou, A.R., Emara, L.H.: Green decoration of graphene oxide Nano sheets with gelatin and gum Arabic for targeted delivery of doxorubicin. Biotechnol. Rep. 34, e00722 (2022)
Dacrory, S., Hashem, A.H., Hasanin, M.: Synthesis of cellulose based amino acid functionalized nano-biocomplex: characterization, antifungal activity, molecular docking and hemocompatibility. Environ. Nanotechnol. Monit. Manag. 15, 100453 (2021)
Huanga, L., Yua, L., Yin, X., Lin, Y., Xu, Y., Niu, Y.: Silver nanoparticles with vanadium oxide nanowires loaded into electrospun dressings for efficient healing of bacterium-infected wounds. J. Colloid Interface Sci. 622, 117–125 (2022)
Hasanin, M., Swielam, E.M., Atwa, N.A., Agwa, M.M.: Novel design of bandages using cotton pads, doped with chitosan, glycogen and ZnO nanoparticles, having enhanced antimicrobial and wounds healing effects. Int. J. Biol. Macromol. 197, 121–130 (2022)
Matter, M.T., Probst, S., Läuchli, S., Herrmann, I.K.: Uniting drug and delivery: metal oxide hybrid nanotherapeutics for skin wound care. Pharmaceutics 12, 780 (2020)
Ferreira, A.M., Mattu, C., Ranzato, E., Ciardelli, G.: Bioinspired porous membranes containing polymer nanoparticles for wound healing. J. Biomed. Mater. Res. A. 102, 4394–4405 (2014)
Zhang, Y., Li, D., Xu, Y., Niu, Y.: Application of a cascaded nanozyme in infected wound recovery of diabetic mice. ACS Biomater. Sci. Eng. 8, 1522–1531 (2022)
Alavi, M., Rai, M.: Topical delivery of growth factors and metal/metal oxide nanoparticles to infected wounds by polymeric nanoparticles: an overview. Expert. Rev. Anti. Infect. Ther. 18, 1021–1032 (2020)
Neuman, M.G., Nanau, R.M., Oruña-Sanchez, L., Coto, G.: Hyaluronic acid and wound healing. J. Pharm. Pharm. Sci. 18, 53–60 (2015)
Graça, M.F.P., Miguel, S.P., Cabral, C.S.D., Correia, I.J.: Hyaluronic acid-based wound dressings: a review. Carbohyd. Polym. 241, 116364 (2020)
Baptista-Silva, S., Borges, S., Costa-Pinto, A.R., Costa, R., Amorim, M., Dias, J.R., Ramos, Ó., Alves, P., Granja, P.L., Soares, R., Pintado, M., Oliveira, A.L.: In situ forming silk sericin-based hydrogel: a novel wound healing biomaterial. ACS Biomater. Sci. Eng. 7, 1573–1586 (2021)
Lamboni, L., Li, Y., Liu, J., Yang, G.: Silk sericin-functionalized bacterial cellulose as a potential wound-healing biomaterial. Biomacromol 17, 3076–3084 (2016)
Yu, Q., Meng, Z., Liu, Y., Li, Z., Sun, X., Zhao, Z.: Photocuring hyaluronic acid/silk fibroin hydrogel containing curcumin loaded chitosan nanoparticles for the treatment of MG-63 cells and ME3T3-E1 cells. Polymers 13, 2302 (2021)
Zhang, M., Wang, D., Ji, N., Lee, S., Wang, G., Zheng, Y., Zhang, X., Yang, L., Qin, Z., Yang, Y.: Bioinspired design of sericin/chitosan/Ag@MOF/GO hydrogels for efficiently combating resistant bacteria, rapid hemostasis, and wound healing. Polymers 13, 2812 (2021)
Bodnár, E., Bakondi, E., Kovács, K., Hegedűs, C., Lakatos, P., Robaszkiewicz, A., Regdon, Z., Virág, L., Szabó, É.: Redox profiling reveals clear differences between molecular patterns of wound fluids from acute and chronic wounds. Oxid. Med. Cell. Longev. 2018, 5286785 (2018)
El-Kafrawy, D.S., Belal, T.S., Mahrous, M.S., Abdel-Khalek, M.M., Abo-Gharam, A.H.: Validated spectrophotometric and rp-hplc–dad methods for the determination of ursodeoxycholic acid based on derivatization with 2-nitrophenylhydrazine. J. AOAC Int. 100, 677–685 (2019)
Jiang, T., Xie, Z., Wu, F., Chen, J., Liao, Y., Liu, L., Zhao, A., Wu, J., Yang, P., Huang, N.: Hyaluronic acid nanoparticle composite films confer favorable time-dependent biofunctions for vascular wound healing. ACS Biomater. Sci. Eng. 5, 1833–1848 (2019)
Choi, K.Y., Chung, H., Min, K.H., Yoon, H.Y., Kim, K., Park, J.H., Kwon, I.C., Jeong, S.Y.: Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials 31, 106–114 (2010)
Verma, J., Kanoujia, J., Parashar, P., Tripathi, C.B., Saraf, S.A.: Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Deliv. Transl. Res. 7, 77–88 (2017)
Wang, Z., Zhang, Y., Zhang, J., Huang, L., Liu, J., Li, Y., Zhang, G., Kundu, S.C., Wang, L.: Exploring natural silk protein sericin for regenerative medicine: an injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 4, 7064 (2014)
Kurihara, T., Noda, Y., Takegoshi, K.: Capping structure of ligand-cysteine on CdSe magic-sized clusters. ACS Omega 4, 3476–3483 (2019)
Huamani-Palomino, R.G., Jacinto, C.R., Alarcón, H., Mejía, I.M., López, R.C., Silva, D.D.O., Cavalheiro, E.T.G., Venâncio, T., Dávalos, J.Z., Valderrama, A.C.: Chemical modification of alginate with cysteine and its application for the removal of Pb(II) from aqueous solutions. Int. J. Biol. Macromol. 129, 1056–1068 (2019)
Ahn, J.S., Choi, H.-K., Lee, K.H., Nahm, J.H., Cho, C.-S.: Novel mucoadhesive polymer prepared by template polymerization of acrylic acid in the presence of silk sericin. J. Appl. Polym. Sci. 80, 274–280 (2001)
Manesa, K.C., Kebede, T.G., Dube, S., Nindi, M.M.: Profiling of silk sericin from cocoons of three southern African wild silk moths with a focus on their antimicrobial and antioxidant properties. Materials (Basel). 13, 5706 (2020)
Carneiro, J., Döll-Boscardin, P.M., Fiorin, B.C., Nadal, J.M., Farago, P.V., Paula, J.P.D.: Development and characterization of hyaluronic acid-lysine nanoparticles with potential as innovative dermal filling. Braz. J. Pharm. Sci. 52, 645–651 (2016)
Weiss, I.M., Muth, C., Drumm, R., Kirchner, H.O.K.: Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 11, 1–5 (2018)
Fallacara, A., Marchetti, F., Pozzoli, M., Citernesi, U.R., Manfredini, S., Vertuani, A.S.: Formulation and characterization of native and crosslinked hyaluronic acid microspheres for dermal delivery of sodium ascorbyl phosphate: a comparative study. Pharmaceutics. 10, 254 (2018)
Lewandowska, K., Sionkowska, A., Grabska, S., Kaczmarek, B.: Surface and thermal properties of collagen/hyaluronic acid blends containing chitosan. Int. J. Biol. Macromol. 92, 371–376 (2016)
Boonpavanitchakul, K., Jarussophon, S., Pimpha, N., Kangwansupamonkon, W., Magaraphan, R.: Silk sericin as a bio-initiator for grafting from synthesis of polylactide via ring-opening polymerization. Eur. Polym. J. 121, 109265 (2019)
Miura, T., Ouchida, R., Yoshikawa, N., Okamoto, K., Makino, Y., Nakamura, T., Morimoto, C., Makino, I., Tanaka, H.: Functional modulation of the glucocorticoid receptor and suppression of nf-kappaB-dependent transcription by ursodeoxycholic acid. J. Biol. Chem. 276, 47371–47378 (2001)
El-Hamoly, T., Abd-El-Rahman, S.S., Al-Abyad, M.: Potential effects of ursodeoxycholic acid on accelerating cutaneous wound healing. PLoS ONE 14, e0226748 (2019)
Balsamo, R., Lanata, L., Egan, C.G.: Mucoactive drugs. Eur. Respir. Rev. 19, 127–133 (2010)
Aldini, G., Altomare, A., Baron, G., Vistoli, G., Carini, M., Borsani, L., Sergio, F.: N-acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radical Res. 52, 751–762 (2018)
Litwiniuk, M., Krejner, A., Speyrer, M.S., Gauto, A.R., Grzela, T.: Hyaluronic acid in inflammation and tissue regeneration. Wounds 28, 78–88 (2016)
Rios de la Rosa, J.M., Pingrajai, P., Pelliccia, M., Spadea, A., Lallana, E., Gennari, A., Stratford, I.J., Rocchia, W., Tirella, A., Tirelli, N.: Binding and internalization in receptor-targeted carriers: the complex role of cd44 in the uptake of hyaluronic acid-based nanoparticles (sirna delivery). Adv. Healthc. Mater. 8, 1901182 (2019)
Jiang, H., Peterson, R.S., Wang, W., Bartnik, E., Knudson, C.B., Knudson, W.: A requirement for the cd44 cytoplasmic domain for hyaluronan binding, pericellular matrix assembly, and receptor-mediated endocytosis in COS-7 cells. J. Biol. Chem. 277, 10531–10538 (2002)
Ersel, M., Uyanikgil, Y., Karbek-Akarca, F., Ozcete, E., Altunci, Y.A., Karabey, F., Cavusoglu, T., Meral, A., Yigitturk, G., Oyku-Cetin, E.: Effects of silk sericin on incision wound healing in a dorsal skin flap wound healing rat model. Med. Sci. Monit. 22, 1064–1078 (2016)
Aramwit, P., Sangcakul, A.: The effects of sericin cream on wound healing in rats. Biosci. Biotechnol. Biochem. 71, 2473–2477 (2007)
Chlapanidas, T., Faragò, S., Lucconi, G., Perteghella, S., Galuzzi, M., Mantelli, M., Avanzini, M.A., Tosca, M.C., Marazzi, M., Vigo, D., Torre, M.L., Faustini, M.: Sericins exhibit ros-scavenging, anti-tyrosinase, anti-elastase, and in vitro immunomodulatory activities. Int. J. Biol. Macromol. 58, 47–56 (2013)
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) (Grant Nos. 2021R1I1A3059994, 221C000142, 2020R1A6A1AA03044512, 2020K1A3A1A19088873, and 2020R1A2C1012586) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through High Value-added Food Technology Development Program funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (321027-5). The authors are also thankful to Core Research Support Center for Natural Products and Medical Materials (CRCNM) in Yeungnam University, South Korea for instrumentation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sood, A., Bhaskar, R., Won, S.Y. et al. Disulfide bond-driven hyaluronic acid/sericin nanoparticles for wound-healing application. J Nanostruct Chem 13, 463–480 (2023). https://doi.org/10.1007/s40097-022-00505-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-022-00505-1