Abstract
Purpose
Alzheimer’s disease (AD) is a multifaceted neurodegenerative disease. To target simultaneously multiple pathological processes involved in AD, natural-origin compounds with unique characteristics are promising scaffolds to develop novel multi-target compounds in the treatment of different neurodegenerative disease, especially AD. In this study, novel chromone-lipoic acid hybrids were prepared to find a new multifunctional lead structure for the treatment of AD.
Methods
Chromone-lipoic acid hybrids were prepared through click reaction and their neuroprotection and anticholinesterase activity were fully evaluated. The anti-amyloid aggregation, antioxidant and metal-chelation activities of the best compound were also investigated by standard methods to find a new multi-functional agent against AD.
Results
The primary biological screening demonstrated that all compounds had significant neuroprotection activity against H2O2-induced cell damage in PC12 cells. Compound 19 as the most potent butyrylcholinesterase (BuChE) inhibitor (IC50 = 7.55 μM) having significant neuroprotection activity as level as reference drug was selected for further biological evaluations. Docking and kinetic studies revealed non-competitive mixed-type inhibition of BuChE by compound 19. It could significantly reduce formation of the intracellular reactive oxygen species (ROS) and showed excellent reducing power (85.57 mM Fe+2), comparable with quercetin and lipoic acid. It could also moderately inhibit Aβ aggregation and selectively chelate with copper ions in 2:1 M ratio.
Conclusion
Compound 19 could be considered as a hopeful multifunctional agent for the further development gainst AD owing to the acceptable neuroprotective and anti-BuChE activity, moderate anti-Aβ aggregation activity, outstanding antioxidant activity as well as selective copper chelation ability.
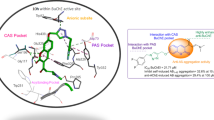
Graphical abstract

A new chromone–lipoic acid hybrid was synthesized as anti-Alzheimer agent with BuChE inhibitory activity, anti-Aβ aggregation, metal-chelation and antioxidant properties.







Similar content being viewed by others
References
Querfurth HW, Laferla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–44.
Kumar A, Ekavali AS. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67:195–203.
Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14:450–64.
Zhang FQ, Jiang JL, Zhang JT, Niu H, Fu XQ, Zeng LL. Current status and future prospects of stem cell therapy in Alzheimer’s disease. Neural Regen Res. 2020;15:242–50.
Nilsson P, Iwata N, Muramatsu S, Tjernberg LO, Winblad B, Saido TC. Gene therapy in Alzheimer’s disease-potential for disease modification. J Cell Mol Med. 2010;14:741–57.
Mushtaq G, Greig NH, Khan JA, Kamal MA. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets. 2014;13:1432–9.
Darvesh S, Cash MK, Reid GA, Martin E, Mitnitski A, Geula C. Butyrylcholinesterase is associated with β-amyloid plaques in the transgenic APPSWE/PSEN1dE9 mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2012;71:2–14.
Podoly E, Hanin G, Soreq H. Alanine-to-threonine substitutions and amyloid diseases: butyrylcholinesterase as a case study. Chem Biol Interact. 2010;187:64–71.
Liu Z, Zhang A, Sun H, Han Y, Kong L, Wang X. Two decades of new drug discovery and development for Alzheimer’s disease. RSC Adv. 2017;7:6046–58.
Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51:347–72.
Jesus Oset-Gasque M, Marco-Contelles J. Alzheimer’s disease, the “One-Molecule, One-Target” paradigm, and the multitarget directed ligand approach. ACS Chem Neurosci. 2018;9:401–3.
Wenzel TJ, Klegeris A. Novel multi-target directed ligand-based strategies for reducing neuroinflammation in Alzheimer’s disease. Life Sci. 2018;15:314–22.
Blaikie L, Kay G, Thoo Lin PK. Current and emerging therapeutic targets of alzheimer’s disease for the design of multi-target directed ligands. Med Chem Commun. 2019;10:2052–72.
Ibrahim MM, Gabr MT. Multitarget therapeutic strategies for Alzheimer’s disease. Neural Regen Res. 2019;14(3):437–40.
Jalili-Baleh L, Nadri H, Forootanfar H, Samzadeh-Kermani A, Tüylü Küçükkılınç T, Ayazgok B, Rahimifard M, Baeeri M, Doostmohammadi M, Firoozpour L, et al. Novel 3-phenylcoumarin–lipoic acid conjugates as multi-functional agents for potential treatment of Alzheimer’s disease. Bioorg Chem. 2018a;79:223–34.
Jalili-Baleh L, Forootanfar H, Tüylü Küçükkılınç T, Nadri H, Abdolahi Z, Ameri A, Jafari M, Ayazgok B, Baeeri M, Rahimifard M, SNA B, et al. Design, synthesis and evaluation of novel multi-target-directed ligands for treatment of Alzheimer’s disease based on coumarin and lipoic acid scaffolds. Eur J Med Chem. 2018b;152:600–14.
de Freitas SM, Dias KST, Gontijo VS, Ortiz CJC, Viegas C Jr. Multi-target directed drugs as a modern approach for drug design towards Alzheimer’s disease: an update. Curr Med Chem. 2018;25:3491–525.
Keri RS, Quintanova C, Chaves S, Silva DF, Cardoso SM, Santos MA. New tacrine hybrids with natural-based cysteine derivatives as multi targeted drugs for potential treatment of Alzheimer’s disease. Chem Boil Drug Des. 2016a;87:101–11.
Choudhary S, Kumar Singh P, Verma H, Singh H, Silakari O. Success stories of natural product-based hybrid molecules for multifactorial diseases. Eur J Med Chem. 2018b;151:62–97.
Reis J, Gaspar A, Milhazes N, Borges F. Chromone as a privileged scaffold in drug discovery: recent advances. J Med Chem. 2017;60(19):7941–57.
Pachón-Angona I, Refouvelet B, Andrýs R, Martin H, Luzet V, Iriepa I, Moraleda I, Diez-Iriepa D, Oset-Gasque MJ, Marco-Contelles J, Musilek K, Ismaili L. Donepezil+chromone+melatonin hybrids as promising agents for Alzheimer’s disease therapy. J Enzy Inhib Med Chem. 2019;34(1):479–89.
Kumar S, Mishra A, Pandey AK. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Compl Altern Med. 2013;13:120.
Kumar S, Pandey AK. Phenolic content, reducing power and membrane protective activities of Solanum xanthocarpum root extracts. Int J Plant Res. 2013;26:301–7.
Leopoldini M, Russo N, Chiodo S, Toscano M. Iron chelation by the powerful antioxidant flavonoid quercetin. J Agricul Food Chem. 2006;54:6343–51.
Reis J, Cagide F, Valencia ME, Teixeira J, Bagetta D, Pérez C, Uriarte E, Oliveira PJ, Ortuso F, Alcaro S, Rodríguez-Franco MI, Borges F. Multi-target-directed ligands for Alzheimer’s disease: discovery of chromone-based monoamine oxidase/cholinesterase inhibitors. Eur J Med Chem. 2018;158:781–800.
Cruz I, Puthongking P, Cravo S, et al. Xanthone and flavone derivatives as dual agents with acetylcholinesterase inhibition and antioxidant activity as potential anti-alzheimer agents. J Chem. 2017;2017:e8587260.
Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JPE. The neuroprotective potential of flavonoids: a multiplicity of effects. Gene Nutr. 2008;3:115–26.
Nabavi SF, Braidy N, Habtemariam S, Orhan IE, Daglia M, Manayi A, Gortzi O, Nabavi SM. Neuroprotective effects of chrysin: from chemistry to medicine. Neurochem Int. 2015;90:224–31.
Gautam R, Jachak SM, Kumar V, Mohan CG. Synthesis, biological evaluation and molecular docking studies of stellatin derivatives a cyclooxygenase (COX-1, COX-2) inhibitors and anti-inflammatory agents. Bioorg Med Chem Lett. 2011;21:1612–6.
Nesi G, Chen Q, Sestito S, Digiacomo M, Yang X, Wang S, Pi R, Rapposelli S. Nature-based molecules combined with rivastigmine: a symbiotic approach for the synthesis of new agents against Alzheimer’s disease. Eur J Med Chem. 2017;141:232–9.
Iida A, Usui T, Zar Kalai F, Han J, Isoda H, Nagumo Y. Protective effects of Nitraria retusa extract and its constituent isorhamnetin against amyloid binduced cytotoxicity and amyloid b aggregation. Biosci Biotechnol Biochem. 2015;79:1548–51.
Fernandez-Bachiller MI, Perez C, Monjas L, Rademann J, Rodriguez-Franco MI. New tacrine-4-oxo-4H-chromene hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with cholinergic, antioxidant, and β-amyloid-reducing properties. J Med Chem. 2012;55:1303–17.
Moini H, Packer L, Saris NEL. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90.
Holmquist L, Stuchbury G, Berbaum K, Muscat S, Young S, Hager K, Engel J, Münch G. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacol Ther. 2007;113:154–64.
Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J, Carlson DA, Münch G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Ad Drug Deliv Rev. 2008;60:1463–70.
Quinn JF, Bussiere JR, Hammond RS, Montine TJ, Henson E, Jones RE, Stackman RW Jr. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007;28(2):213–25.
Rosini M, Andrisano V, Bartolini M, Bolognesi ML, Hrelia P, Minarini A, Tarozzi A, Melchiorre C. Rational approach to discover multipotent anti-Alzheimer drugs. J Med Chem. 2005;48:360–3.
Sanga Z, Lia Y, Qiang X, Xiao G, Liu Q, Tan Z, Deng Y. Multifunctional scutellarin–rivastigmine hybrids with cholinergic, antioxidant, biometal chelating and neuroprotective properties for the treatment of Alzheimer’s disease. Bioorg Med Chem. 2015;23:668–80.
Airoldi C, La Ferla B, D’Orazio G, Ciaramelli C. Palmioli A flavonoids in the treatment of Alzheimer’s and other neurodegenerative diseases. Curr Med Chem. 2018;25:3228–46.
Jalili-Baleh L, Babaei E, Abdpour S, Bukhari SNA, Foroumadi A, Ramazani A, Sharifzadeh M, Abdollahi M, Khoobi M. A review on flavonoid-based scaffolds as multi-target-directed ligands (MTDLs) for Alzheimer’s disease. Eur J Med Chem. 2018;152:570–89.
Melagraki G, Afantitis A, Igglessi-Markopoulou O, Detsi A, Koufaki M, Kontogiorgis C, Hadjipavlou-Litina DJ. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur J Med Chem. 2009;44:3020–6.
Hager K, Marahrens A, Kenklies M, Riederer P, Munch G. R-Lipoic acid as a new treatment option for Alzheimer type dementia. Arch Gerontol Geriatr. 2001;32:275–82.
Sang Z, Wang K, Shi J, Liu W, Cheng X, Zhu G, Wang Y, Zhao Y, Qiao Z, Wu A, Tan Z. The development of advanced structural framework as multi-target-directed ligands for the treatment of Alzheimer’s disease. Eur J Med Chem. 2020;192:112180.
Bolognesi ML, Rosini M, Andrisano V, Bartolini M, Minarini A, Tumiatti V, Melchiorre C. MTDL design strategy in the context of Alzheimer’s disease: from lipocrine to memoquin and beyond. Curr Pharm Des. 2009;15:601–13.
Agalave SG, Maujan SR, Pore VS. Click chemistry: 1,2,3-Triazoles as pharmacophores. Chem Asian J. 2011;6:2696–718.
Xu M, Peng Y, Zhu L, Wang S, Ji J, Rakesh KP. Triazole derivatives as inhibitors of Alzheimer’s disease: current developments and structure-activity relationships. Eur J Med Chem. 2019;180:656–72.
Rastegari A, Nadri H, Mahdavi M, Moradi A, Mirfazli SS, Edraki N, Moghadam FH, Larijani B, Akbarzadeh T, Saeedi M. Design, synthesis and anti-Alzheimer’s activity of novel 1,2,3-triazole-chromenone carboxamide derivatives. Bioorg Chem. 2019;83:391–401.
Naik MM, Tilve SG. Pyrrolidine and iodine catalyzed domino aldol-Michael-dehydrogenative synthesis of flavones. Tetrahedron Lett. 2014;55:3340–3.
Sagrera G, Bertucci A, Vazquez A, Seoane G. Synthesis and antifungal activities of natural and synthetic bioflavonoids. Bioorg Med Chem. 2011;19:3060–73.
Jaen JC, Wise LD, Heffner TG, Pugsley TA, Meltzer LT. Dopamine auto receptor agonists as potential antipsychotics. 2. (Aminoalkoxy)-4H-1-benzopyran-4-ones. J Med Chem. 1991;34:248–56.
Luo W, Su YB, Hong C, Tian RG, Su LP, Wang YQ, Li Y, Yue JJ, Wang CJ. Design, synthesis and evaluation of novel 4-dimethylamine flavonoid derivatives as potential multifunctional anti-Alzheimer agents. Bioorg Med Chem. 2013;21:7275–82.
Wu T, Zhang Q, Hu J, Zhang G, Liu S. Composite silica nanospheres covalently anchored with gold nanoparticles at the outer periphery of thermoresponsive polymer brushes. J Mater Chem. 2012;22:5155–63.
Garin D, Oukhatar F, Mahon AB, Try AC, Dubois-Dauphin M, Laferla FM, Demeunynck M, Sallanon MM, Chierici S. Proflavine derivatives as fluorescent imaging agents of amyloid deposits. Bioorg Med Chem Lett. 2011;21:2203–6.
Zsolt D, Juhász A, Gálfi M, Soós K, Papp R, Zádori D, Penke B. Method for measuring neurotoxicity of aggregating polypeptides with the MTT assay on differentiated neuroblastoma. Cells Brain Res Bull. 2003;62:223–9.
Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95.
Levine H. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptide: detection of amyloid aggregation in solution. Protein Sci. 1993;20:404–10.
Baeeri M, Momtaz S, Navaei-Nigjeh M, Niaz K, Rahimifard M, GhasemiNiri SF, Sanadgol N, Hodjat M, Sharifzadeh M, Abdollahi M. Molecular evidence on the protective effect of ellagic acid on phosaloneinduced senescence in rat embryonic fibroblast cells. Food Chem Toxicol. 2017;100:8–23.
Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27.
Pazik A, Skwierawska A. Synthesis and spectroscopic properties of new bis-tetrazoles. J Incl Phenom Macrocycl Chem. 2013;77:83–94.
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31:455–61.
Dassault Systèmes BIOVIA. Discovery studio modeling, release, 4. San Diego: Dassault Systemes; 2015.
Acknowledgments
This work was supported by a grant from The National Institute for Medical Research Development (NIMAD, grant number: 971370).
Author information
Authors and Affiliations
Contributions
Leili Jalili-Baleh synthesized and characterized the compounds and participated in the writing of the manuscript. Hamid Nadri evaluated ChEs inhibition activity of the compounds, docking and kinetic studies. Hamid Forootanfar evaluated neuroprotective activity of the compounds. Tuba Tüylü Küçükkılınç and Beyza Ayazgök participated in intracellular ROS inhibition activity of the compound. Mahban Rahimifard and Maryam Baeeri performed FRAP assay. Mohammad Sharifzadeh, Mohammad Abdollahi and Alireza Foroumadi contributed to the characterization of the compounds, data analysis and revision of the manuscript. Mehdi Khoobi conceived the main idea of the study, organized the work and contributed to the overall analysis of the results. All the authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 7351 kb)
Rights and permissions
About this article
Cite this article
Jalili-Baleh, L., Nadri, H., Forootanfar, H. et al. Chromone–lipoic acid conjugate: Neuroprotective agent having acceptable butyrylcholinesterase inhibition, antioxidant and copper-chelation activities. DARU J Pharm Sci 29, 23–38 (2021). https://doi.org/10.1007/s40199-020-00378-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-020-00378-1