Abstract
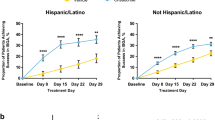
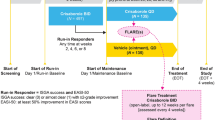
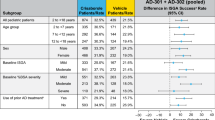
Crisaborole ointment 2% (Eucrisa™) is a novel, anti-inflammatory inhibitor of phosphodiesterase 4 (PDE4) that is available in the USA for the topical treatment of mild to moderate atopic dermatitis in patients aged ≥ 2 years. In two short-term (28 days), identically designed, multicentre, phase III studies in this patient population, topical therapy with crisaborole ointment 2% reduced disease severity and pruritus severity compared with vehicle, with the effect established early and sustained over the course of treatment. Improvements in the other signs of atopic dermatitis (erythema, exudation, excoriation, induration/papulation, and lichenification) were also seen. Crisaborole ointment 2% was generally well tolerated in the short-term studies, with its favorable safety profile maintained over the longer term (up to 52 weeks) in a multicentre, extension study. Most treatment-emergent adverse events (TEAEs) were of mild to moderate severity and considered unrelated to the study medication. Moreover, the incidence of application-site pain following short- and longer-term topical therapy with crisaborole ointment 2% was low. In conclusion, crisaborole ointment 2% is an effective and generally well tolerated new topical option for the management of mild to moderate atopic dermatitis in patients aged ≥ 2 years, with the potential to effectively treat this patient population over the longer term without the safety concerns associated with other current topical anti-inflammatory agents.
Similar content being viewed by others
References
Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–51.
Ellis CN, Mancini AJ, Paller AS, et al. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(3 Suppl):S18–22.
Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22(2):125–37.
Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49–57.
Schneider L, Tilles S, Lio P, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013;131(2):295–9 (e1–27).
Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15(4):303–10.
Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32.
Eichenfield LF, Friedlander SF, Simpson EL, et al. Assessing the new and emerging treatments for atopic dermatitis. Semin Cutan Med Surg. 2016;35(5 Suppl):S92–6.
Wang D, Beck LA. Immunologic targets in atopic dermatitis and emerging therapies: an update. Am J Clin Dermatol. 2016;17(5):425–43.
Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of TH2/TH22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130(6):1344–54.
Akama T, Baker SJ, Zhang Y-K, et al. Discovery and structure-activity study of a novel benzoxaborole anti-inflammatory agent (AN2728) for the potential topical treatment of psoriasis and atopic dermatitis. Bioorg Med Chem Lett. 2009;19(8):2129–32.
Anacor Pharmaceuticals Inc. EUCRISA™ (crisaborole) ointment, 2%, for topical use: US prescribing information. 2016. http://www.fda.gov/. Accessed 25 Sep 2017.
Freund YR, Akama T, Alley MR, et al. Boron-based phosphodiesterase inhibitors show novel binding of boron to PDE4 bimetal center. FEBS Lett. 2012;586(19):3410–4.
Zane LT, Hughes MH, Shakib S. Tolerability of crisaborole ointment for application on sensitive skin areas: a randomized, double-blind, vehicle-controlled study in healthy volunteers. Am J Clin Dermatol. 2016;17(5):519–26.
Zane LT, Kircik L, Call R, et al. Crisaborole topical ointment, 2% in patients ages 2 to 17 years with atopic dermatitis: a phase 1b, open-label, maximal-use systemic exposure study. Pediatr Dermatol. 2016;33(4):380–7.
Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75(3):494–503.
Fowler J, Cook-Bolden F, Pariser D, et al. Crisaborole ointment improves global atopic dermatitis severity: pooled results from two phase 3 clinical trials [abstract no. 5667 plus oral presentation]. In: 75th Annual Meeting of the American Academy of Dermatology. 2017.
Guttman-Yassky E, Hanifin J, Murell D, et al. Crisaborole ointment provides early relief of pruritus regardless of baseline disease severity in two phase 3 clinical trials in patients with mild or moderate AD [abstract no. 5571 plus oral presentation]. In: 75th Annual Meeting of the American Academy of Dermatology. 2017.
US FDA Center for Drug Evaluation and Research. EUCRISA™ (crisaborole) ointment, 2%: medical review(s). 2015. http://www.fda.gov/. Accessed 25 Sep 2017.
Eichenfield LF, Call RS, Forsha D, et al. Long-term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. J Am Acad Dermatol. 2017;77(4):641–9.e5.
Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156(2):203–21.
Acknowledgements
During the peer review process, the manufacturer of crisaborole ointment 2% was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Sheridan Hoy is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/DA98F0600F347AE8.
Additional information
The manuscript was reviewed by: M. G. Lebwohl, Department of Dermatology, The Icahn School of Medicine at Mount Sinai, New York, NY, USA; L. Stein Gold, Department of Dermatology, Henry Ford Hospital, Detroit, MI, USA.
Rights and permissions
About this article
Cite this article
Hoy, S.M. Crisaborole Ointment 2%: A Review in Mild to Moderate Atopic Dermatitis. Am J Clin Dermatol 18, 837–843 (2017). https://doi.org/10.1007/s40257-017-0327-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-017-0327-4