Abstract
Objective
The objective of this study was to evaluate the efficacy and safety of intravenous vinpocetine administration as part of a comprehensive treatment for acute cerebral infarction in a Chinese population.
Methods
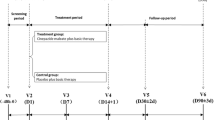
610 acute cerebral infarction patients were randomized into two groups: the vinpocetine group (469 patients) received cytidine disphosphate choline 0.4–0.5 g in combination with aspirin 75–100 mg or clopidogrel 75 mg once daily, plus vinpocetine 30 mg intravenously once daily for 7 days, while the control group (141 patients) received cytidine disphosphate choline 0.4–0.5 g in combination with aspirin 75–100 mg or clopidogrel 75 mg once daily for 7 days. Additionally, patients received medications for symptoms such as hypertension, hyperglycemia, hyperlipidemia, and intracranial hypertension when necessary. Mini-Mental State Examination (MMSE), National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale, and Barthel Index (BI) scores and transcranial doppler (TCD) were assessed at baseline, 7, 14, and 90 days after treatment. Adverse events (AEs) and abnormalities in blood, urine, liver, and kidney function were monitored.
Results
MMSE, NIHSS, and BI scores were significantly higher in the vinpocetine group than in the control group 90 days after treatment, indicating significantly improved cognitive skill, neurological function, and quality of life (QOL) in the vinpocetine group versus the control group. Importantly, such effects of vinpocetine were maintained over time. In addition, TCD monitoring showed significantly increased cerebral blood flow associated with vinpocetine versus control. No significant difference in safety was noted between the two groups.
Conclusions
When used as part of treatment for acute cerebral infarction, vinpocetine improves patients’ cerebral blood flow, cognitive quality, neurological functions, and QOL. Vinpocetine could be an effective and safe component of treatment regimen for acute cerebral infarction.
Similar content being viewed by others
References
Bansal S, Sangha KS, Khatri P. Drug treatment of acute ischemic stroke. Am J Cardiovasc Drugs. 2013;13:57–69.
Venketasubramanian N, Chen CL, Gan RN, et al. A double-blind, placebo-controlled, randomized, multicenter study to investigate CHInese Medicine Neuroaid Efficacy on Stroke recovery (CHIMES study). Int J Stroke. 2009;4:54–60.
Ng PP, Higashida RT, Cullen SP, et al. Intraarterial thrombolysis trials in acute ischemic stroke. J Vasc Interv Radiol. 2004;15:S77–85.
Bereczki D, Fekete I. Vinpocetine for acute ischaemic stroke. Cochrane Database Syst Rev. 2008;1:CD000480.
Patyar S, Prakash A, Modi M, et al. Role of vinpocetine in cerebrovascular diseases. Pharmacol Rep. 2011;63:618–28.
Cai Y, Li JD, Yan C. Vinpocetine attenuates lipid accumulation and atherosclerosis formation. Biochem Biophys Res Commun. 2013;434:439–43.
Tamaki N, Matsumoto S. Agents to improve cerebrovascular circulation and cerebral metabolism–vinpocetine. Nihon Rinsho. 1985;43:376–8.
Szobor A, Klein M. Ethyl apovincaminate therapy in neurovascular diseases. Arzneimittelforschung. 1976;26:1984–9.
Bagoly E, Fehér G, Szapáry L. The role of vinpocetine in the treatment of cerebrovascular diseases based in human studies. Orv Hetil. 2007;148:1353–8.
Burtsev EM, Savkov VS, Shprakh VV, et al. 10-year experience with using Cavinton in cerebrovascular disorders. Zh Nevropatol Psikhiatr Im S S Korsakova. 1992;92:56–60.
Szapáry L, Horváth B, Alexy T, et al. Effect of vinpocetin on the hemorheologic parameters in patients with chronic cerebrovascular disease. Orv Hetil. 2003;144:973–8.
Tabeeva GR. Azimova IuE. The multimodal strategy for the neuroprotection in stroke: results of the Russian multicenter clinical-epidemiological program SOKOL [in Russian]. Zh Nevrol Psikhiatr Im S S Korsakova. 2012;112:20–30.
Feigin VL, Doronin BM, Popova TF, et al. Vinpocetine treatment in acute ischaemic stroke: a pilot single-blind randomized clinical trial. Eur J Neurol. 2001;8:81–5.
Overgaard K. The effects of citicoline on acute ischemic stroke: a review. J Stroke Cerebrovasc Dis. 2014;23:1764–9.
Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10:839–46.
Shi PY, Zhou XC, Yin XX, et al. Early application of citicoline in the treatment of acute stroke: a meta-analysis of randomized controlled trials. J Huazhong Univ Sci Technolog Med Sci. 2016;36(2):270–7.
Zhu Y, Zhang G, Zhao J, et al. Efficacy and safety of mildronate for acute ischemic stroke: a randomized, double-blind, active-controlled phase II multicenter trial. Clin Drug Investig. 2013;33:755–60.
Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30:1538–41.
Dávalos A, Alvarez-Sabín J, Castillo J, et al. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet. 2012;380:349–57.
Mitta M, Goel D, Bansal KK, et al. Edaravone—citicoline comparative study in acute ischemic stroke (ECCS-AIS). J Assoc Physicians India. 2012;60:36–8.
Bustamante A, Giralt D, Garcia-Bonilla L, et al. Citicoline in pre-clinical animal models of stroke: a meta-analysis shows the optimal neuroprotective profile and the missing steps for jumping into a stroke clinical trial. J Neurochem. 2012;123:217–25.
Dávalos A, Castillo J, Alvarez-Sabín J, et al. Oral citicoline in acute ischemic stroke: an individual patient data pooling analysis of clinical trials. Stroke. 2002;33:2850–7.
Wang Q, Ye H, Su Y. Transcranial Doppler sonography monitors cerebral blood flow of mannitol-treated patients with acute large hemispheric infarction. Turk Neurosurg. 2014;24:333–6.
Katsura K, Suda S, Abe A, et al. Brain protection therapy in acute cerebral infarction. J Nippon Med Sch. 2012;79:104–10.
Han SW, Lee SS, Kim SH, et al. Effect of cilostazol in acute lacunar infarction based on pulsatility index of transcranial Doppler (ECLIPse): a multicenter, randomized, double-blind, placebo-controlled trial. Eur Neurol. 2013;69:33–40.
Shanghai Rxmidas Pharmaceuticals Co. Ltd. Chinese Assessment for Vinpocetine in Neurology (CAVIN) [ClinicalTrials.gov identifier NCT01400035]. US National Institutes of Health, ClinicalTrials.gov. Accessed 20 May 2016.
Acknowledgments
We would like to thank the Hungarian Embassy in China for their support of this study. We would further like to thank Beijing Military General Hospital, Peking University First Hospital, Beijing Jishuitan Hospital, Shengjing Hospital of China Medical University, The Second Xiangya Hospital of Central South University, The Second Hospital of Jilin University, The Fourth Hospital of Harbin Medical University, Tianjin Huanhu Hospital, The Second Affiliated Hospital of Kunming Medical University, The People’s Hospital of Liaoning Province, Beijing Haidian Hospital, Tianjin Hospital of Chinese Traditional and Western Medicine, Tianjin Fifth Central Hospital, Xi’an Central Hospital, Wuxi No. 2 People’s Hospital, The People’s Hospital of Lvshunkou Dalian, and Anshan Changda Hospital for their participation in and support of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study is partly funded by the Chinese-Hungarian Scientific Cooperation Fund supported by the Hungary Embassy in China. The funding source did not play any role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflict of interest
Weiwei Zhang, Yining Huang, Ying Li, Liming Tan, Jianfei Nao, Hongtao Hu, Jingyu Zhang, Chen Li, Yuenan Kong, and Yulin Song declare no conflicts of interest.
Ethical approval
All procedures in this study were in accordance with the 1964 Helsinki declaration and its amendments and were approved by the institutional review board of each participating hospital.
Informed consent
Written informed consent was obtained from all of the enrolled patients.
Rights and permissions
About this article
Cite this article
Zhang, W., Huang, Y., Li, Y. et al. Efficacy and Safety of Vinpocetine as Part of Treatment for Acute Cerebral Infarction: A Randomized, Open-Label, Controlled, Multicenter CAVIN (Chinese Assessment for Vinpocetine in Neurology) Trial. Clin Drug Investig 36, 697–704 (2016). https://doi.org/10.1007/s40261-016-0415-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0415-x