Abstract
Background
Several disease-modifying therapies (DMTs) treat relapsing–remitting multiple sclerosis (RRMS) and primary progressive multiple sclerosis (PPMS). Few comprehensive cost-effectiveness analyses exist in this area, particularly from a payer perspective, despite rapidly increasing prices of DMTs.
Objective
We aimed to systematically compare cost effectiveness of all relevant DMTs for first-line treatment of RRMS, second-line treatment of RRMS, and first-line treatment of PPMS.
Methods
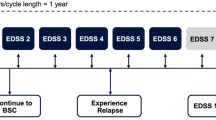
We used a Markov model with health states based on Expanded Disability Status Score categories. Upon discontinuing first-line treatment, RRMS patients continued to second-line therapy then to supportive care, and PPMS patients moved directly to supportive care. Data was sourced from clinical trials and commercially and publicly available sources. The target population was treatment-naïve adults with RRMS or PPMS. We used a lifetime horizon from a US payer perspective, and compared DMTs for RRMS (first-line: dimethyl fumarate, glatiramer acetate, interferon β-1a, interferon β-1b, peginterferon β-1a, teriflunomide, natalizumab, fingolimod, and ocrelizumab; second-line: alemtuzumab, natalizumab, fingolimod, and ocrelizumab), ocrelizumab for PPMS, and supportive care. Outcome measures included total costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs).
Results
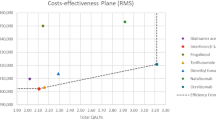
For RRMS first-line therapy, ocrelizumab dominated the other DMTs with an ICER of US$166,338/QALY compared with supportive care. For RRMS second-line therapy, alemtuzumab dominated the other three DMTs, providing more QALYs for lower costs. For PPMS, ocrelizumab had an ICER of US$648,799/QALY compared with supportive care. Wide variability in results was observed in the probabilistic sensitivity analysis. Results were sensitive to the relative risk of progression and cost of DMTs.
Conclusions
Ocrelizumab would likely be cost effective as a first-line treatment for RRMS with a discounted price but was not cost effective for PPMS. Alemtuzumab dominated other options for second-line treatment of RRMS. Other DMTs were generally similar in terms of costs and health outcomes, providing health benefits compared to supportive care but with significant added costs. If drug prices were lowered, more DMTs could be cost effective.



Similar content being viewed by others
References
Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pa. Arch Phys Med Rehabil. 2014;95:986–995.e1.
Piccinni C, Ronconi G, Calabria S, Dondi L, Forcesi E, Rossi E, et al. Healthcare resources utilisation in primary progressive multiple sclerosis. Neurol. Sci. 2018;39:1169–74.
Goodin DS. The epidemiology of multiple sclerosis: insights to disease pathogenesis. Handb Clin Neurol. 2014;122:231–66.
Bakalar N. Multiple sclerosis drug doesn’t prevent onset of disability, study finds [Internet]. New York Times. 2012 [cited 2018 Jul 18]. http://www.nytimes.com/2012/07/18/health/research/multiple-sclerosis-drug-doesnt-stop-disability-study-finds.html. Accessed 18 Jul 2018.
National Multiple Sclerosis Society. FDA approves Ocrevus™ (ocrelizumab) for people with primary progressive MS or relapsing MS—first disease-modifying therapy for primary progressive MS [Internet]. 2017 [cited 2018 Jul 18]. http://www.nationalmssociety.org/About-the-Society/News/FDA-Approves-Ocrevus. Accessed 18 Jul 2018.
Office of the Attourey General Commonwealth of Massachusetts. Examination of health care cost trends and cost drivers pursuant to G.L. c. 12C, § 17 [Internet]. 2016 [cited 2018 Jul 18]. https://www.mass.gov/files/documents/2016/10/ts/cc-market-101316.pdf. Accessed 18 Jul 2018.
National Multiple Sclerosis Society. Make MS medications accessible [Internet]. [cited 2018 Jul 18]. https://www.nationalmssociety.org/Treating-MS/Medications/Make-MS-Medications-Accessible. Accessed 18 Jul 2018.
Agashivala N, Kim E. Cost-effectiveness of early initiation of fingolimod versus delayed initiation after 1 year of intramuscular interferon beta-1a in patients with multiple sclerosis. Clin Ther. 2012;34:1583–90.
Chevalier J, Chamoux C, Hammès F, Chicoye A. Cost-effectiveness of treatments for relapsing remitting multiple sclerosis: a French societal perspective. PLoS One. 2016;11:e0150703.
Furneri G, Santoni L, Marchesi C, Iannazzo S, Cortesi P, Piacentini P, et al. Cost-effectiveness analysis of delayed-release dimethyl-fumarate in the treatment of relapsing-remitting multiple sclerosis in Italy. Value Health. 2015;18:A697.
Hernandez L, Guo S, Kinter E, Fay M. Cost-effectiveness analysis of peginterferon beta-1a compared with interferon beta-1a and glatiramer acetate in the treatment of relapsing-remitting multiple sclerosis in the United States. J Med Econ. 2016;19:684–95.
Maruszczak MJ, Montgomery SM, Griffiths MJS, Bergvall N, Adlard N. Cost-utility of fingolimod compared with dimethyl fumarate in highly active relapsing-remitting multiple sclerosis (RRMS) in England. J Med Econ. 2015;6998:1–24.
Mauskopf J, Fay M, Iyer R, Sarda S, Livingston T. Cost-effectiveness of delayed-release dimethyl fumarate for the treatment of relapsing forms of multiple sclerosis in the United States. J Med Econ. 2016;6998:1–11.
Olsen J, Wiren A. Cost-effectiveness of dimethyl fumarate treatment for relapsing-remitting multiple sclerosis from a danish perspective. Value Health. 2015;18:A758.
Raikou M, Kalogeropoulou M, Rombopoulos G. A cost-effectiveness analysis of fingolimod versus dimethyl fumarate as a second-line disease modifying treatment in patients with highly active relapsing-remitting multiple sclerosis. Value Health. 2015;18:A758.
Yamamoto D, Campbell JD. Cost-effectiveness of multiple sclerosis disease-modifying therapies: a systematic review of the literature. Autoimmune Dis. 2012;2012:784364.
Zhang X, Hay JW, Niu X. Cost effectiveness of fingolimod, teriflunomide, dimethyl fumarate and intramuscular interferon-β1a in relapsing-remitting multiple sclerosis. CNS Drugs. 2015;29:71–81.
OmedaRx. Medication Policy Manual, Policy No: dru479, Topic: Ocrevus™, ocrelizumab [Internet]. September. [cited 2018 Jul 18]. http://blue.regence.com/trgmedpol/drugs/dru479.pdf. Accessed 18 Jul 2018.
Blue Cross Blue Shield of Michigan. Prior authorization and step therapy coverage criteria [Internet]. 2018. https://www.bcbsm.com/content/dam/public/Consumer/Documents/help/documents-forms/pharmacy/prior-authorization-and-step-therapy-guidelines.pdf. Accessed 18 Jul 2018.
Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R, GALA Study Group. Three times weekly glatiramer acetate in relapsing-remitting multiple sclerosis. Ann Neurol. 2013;73:705–13.
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med. 2008;358:676–88.
Palace J, Bregenzer T, Tremlett H, Oger J, Zhu F, Boggild M, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open. 2014;4:e004073.
Koch M, Kingwell E, Rieckmann P, Tremlett H. The natural history of primary progressive multiple sclerosis. Neurology. 2009;73:1996–2002.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–52.
Sormani MP, Bonzano L, Roccatagliata L, Mancardi GL, Uccelli A, Bruzzi P. Surrogate endpoints for EDSS worsening in multiple sclerosis. A meta-analytic approach. Neurology. 2010;75:302–9.
Chilcott J, McCabe C, Tappenden P, O’Hagan A, Cooper NJ, Abrams K, et al. Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. Br Med J. 2003;326:522–5.
National Institute for Health and Care Excellence. Dimethyl fumarate for treating relapsing-remitting multiple sclerosis. 2013. https://www.nice.org.uk/guidance/TA320/documents/multiple-sclerosis-relapsingremitting-dimethylfumarate-evaluation-report2. Accessed 18 Jul 2018.
Gani R, Giovannoni G, Bates D, Kemball B, Hughes S, Kerrigan J. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics. 2008;26:617–27.
Su W, Kansal A, Vicente C, Deniz B, Sarda S. The cost-effectiveness of delayed-release dimethyl fumarate for the treatment of relapsing-remitting multiple sclerosis in Canada. J Med Econ. 2016;3:1–10.
Tappenden P, McCabe C, Chilcott J, Simpson E, Nixon R, Madan J, et al. Cost-effectiveness of disease-modifying therapies in the management of multiple sclerosis for the medicare population. Value Heal. 2009;12:657–65.
Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367:1087–97.
Genentech. Data on File.
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–107.
Sanofi Genzyme. Data on File.
Sanofi Genzyme. Teriflunomide US adaptation for AMCP dossiers.
Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–20.
Nickerson M, Cofield SS, Tyry T, Salter AR, Cutter GR, Marrie RA. Impact of multiple sclerosis relapse: the NARCOMS participant perspective. Mult Scler Relat Disord. 2015;4:234–40.
CDC/NCHS National Vital Statistics System. Life table for the total population: United States, 2013. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_03.pdf. Accessed 8 Oct 2016.
Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med. 1997;29:101–6.
Patzold U, Pocklington PR. Course of multiple sclerosis. First results of a prospective study carried out of 102 MS patients from 1976–1980. Acta Neurol Scand. 1982;65:248–66.
Oleen-Burkey M, Castelli-Haley J, Lage MJ, Johnson KP. Burden of a multiple sclerosis relapse. Patient Patient Center Outcomes Res. 2012;5:57–69.
Institute for Clinical and Economic Review. Disease-Modifying Therapies for Relapsing-Remitting and Primary-Progressive Multiple Sclerosis: Effectiveness and Value [Internet]. 2017 [cited 2018 Jul 18]. https://icer-review.org/wp-content/uploads/2016/08/CTAF_MS_Final_Report_030617.pdf. Accessed 18 Jul 2018.
SSR Health. US Brand Rx Net Prices (access restricted document). 2016. https://www.ssrhealth.com/. Accessed 15 Dec 2018.
Havrdova E, Arnold DL, Cohen JA, Hartung H-P, Fox EJ, Giovannoni G, et al. Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology. 2017;89:1107–16.
Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology. 2006;66:1696–702.
Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? WJCC. 2015;3:545–55.
Putzki N, Yaldizli O, Maurer M, Cursiefen S, Kuckert S, Klawe C, et al. Efficacy of natalizumab in second line therapy of relapsing-remitting multiple sclerosis: results from a multi-center study in German speaking countries. Eur J Neurol. 2010;17:31–7.
Institute for Clinical and Economic Review. Overview of the ICER value framework [Internet]. 2017 [cited 2018 Jul 18]. https://icer-review.org/wp-content/uploads/2016/02/ICER-VAF-Update-Proposals-020117.pdf. Accessed 18 Jul 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this study was provided by the Institute for Clinical and Economic Review, Boston, MA, USA.
Conflicts of interest
Marita Zimmermann served as a consultant to Genentech, and Josh Carlson has been a consultant for Genentech, Bayer, Pfizer, and Sandoz Inc., all of whom manufacture products included in this manuscript. These consultancies were on products unrelated to multiple sclerosis or any of the topics covered in this study. All methods, results, and opinions expressed in this manuscript were not influenced by these consultancies. Elizabeth Brouwer, Jeffrey A. Tice, Matt Seidner, Anne M. Loos, Shanshan Liu, Richard H. Chapman, and Varun Kumar have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zimmermann, M., Brouwer, E., Tice, J.A. et al. Disease-Modifying Therapies for Relapsing–Remitting and Primary Progressive Multiple Sclerosis: A Cost-Utility Analysis. CNS Drugs 32, 1145–1157 (2018). https://doi.org/10.1007/s40263-018-0566-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-018-0566-9