Abstract
Background
The effects of tranexamic acid on spontaneous intracerebral hemorrhage in reducing hematoma expansion and mortality as well as its role in thromboembolic complications and in the improvement of functional outcomes remain substantially uncertain.
Objective
The objective of this systematic review was to evaluate the efficacy and safety of tranexamic acid in patients with spontaneous intracerebral hemorrhage.
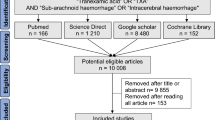
Methods
Several databases were searched from inception up to 20 June, 2021. We included randomized controlled trials that compared tranexamic acid with placebo or no treatment for the management of intracerebral hemorrhage. The primary outcomes were hematoma expansion and 90-day mortality. The secondary outcomes were hemorrhagic volume change, thromboembolic complications, and functional outcomes.
Results
Overall, six trials with 2800 patients were included in this meta-analysis. Tranexamic acid was associated with a reduced risk of hematoma expansion (relative risk 0.87, 95% confidence interval [CI] 0.77–0.99, p = 0.03, I2 = 0%, six trials with 2800 participants) and a lessening of hematoma volume change (mean difference − 1.28, 95% CI − 2.44 to − 0.12; p = 0.03; I2 = 0%, four trials with 2626 participants), without a corresponding higher rate of major thromboembolic complications (relative risk 1.20, 95% CI 0.85–1.69; p = 0.80; I2 = 0%, five trials with 2759 participants). The present analysis also demonstrated that tranexamic acid had no effect on reducing 90-day mortality (relative risk 1.02, 95% CI 0.88–1.19; p = 0.80; I2 = 0%, five trials with 2770 participants).
Conclusions
In adults with spontaneous intracerebral hemorrhage, tranexamic acid reduced the risk of intracerebral hemorrhage growth compared with the control. The effects on 90-day mortality remained inconclusive. Further studies should report death within 24 h and death due to bleeding whenever possible.



Similar content being viewed by others
References
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–44.
van Asch CJJ, Luitse MJA, Rinkel GJE, van der Tweel I, Algra A, Klijn CJM. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76.
Gulati D, Dua D, Torbey MT. Hemostasis in intracranial hemorrhage. Front Neurol. 2017;8:80.
Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–81.
Meretoja A, Yassi N, Wu TY, Churilov L, Sibolt G, Jeng J-S, et al. Tranexamic acid in patients with intracerebral haemorrhage (STOP-AUST): a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2020;19(12):980–7.
Steiner T, Bösel J. Options to restrict hematoma expansion after spontaneous intracerebral hemorrhage. Stroke. 2010;41:402–9.
Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005–32.
Tengborn L, Blomback M, Berntorp E. Tranexamic acid: an old drug still going strong and making a revival. Thromb Res. 2015;135:231–42.
Jokar A, Ahmadi K, Salehi T, Sharif-Alhoseini M, Rahimi-Movaghar V. The effect of tranexamic acid in traumatic brain injury: a randomized controlled trial. Chin J Traumatol. 2017;20:49–51.
Yutthakasemsunt S, Kittiwatanagul W, Piyavechvirat P, Thinkamrop B, Phuenpathom N, Lumbiganon P. Tranexamic acid for patients with traumatic brain injury: a randomized, double-blinded, placebo-controlled trial. BMC Emerg Med. 2013;13:20.
The CRASH-2 Collaborators IBS. Effect of tranexamic acid in traumatic brain injury: a nested randomised, placebo controlled trial (CRASH-2 Intracranial Bleeding Study). BMJ. 2011;343:3795.
Hu W, Xin Y, Chen X, Song Z, He Z, Zhao Y. Tranexamic acid in cerebral hemorrhage: a meta-analysis and systematic review. CNS Drugs. 2019;33:327–36.
Law ZK, Meretoja A, Engelter ST, Christensen H, Muresan EM, Glad SB, et al. Treatment of intracerebral haemorrhage with tranexamic acid: a review of current evidence and ongoing trials. Eur Stroke J. 2017;2:13–22.
Gao B, Xue T, Rong X, Yang Y, Wang Z, Chen Z, et al. Tranexamic acid inhibits hematoma expansion in intracerebral hemorrhage and traumatic brain injury: does blood pressure play a potential role? A meta-analysis from randmized controlled trials. J Stroke Cerebrovasc Dis. 2020;30:105436.
Arumugam A, Na AR, Theophilus SC, Shariffudin A, Abdullah JM. Tranexamic acid as antifibrinolytic agent in non traumatic intracerebral hemorrhages. Malays J Med Sci. 2015;22:62–71.
Sprigg N, Renton CJ, Dineen RA, Kwong Y, Bath PM. Tranexamic acid for spontaneous intracerebral hemorrhage: a randomized controlled pilot trial (ISRCTN50867461). J Stroke Cerebrovasc Dis. 2014;23:1312–8.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-94.
Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5.
Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–6.
Papaioannou D, Brazier J, Paisley S. Systematic searching and selection of health state utility values from the literature. Value Health. 2013;16:686–95.
Kim S, Won CW, Kim BS, Kim S, Yoo J, Byun S, et al. EuroQol Visual Analogue Scale (EQ-VAS) as a predicting tool for frailty in older Korean adults: the Korean Frailty an Aging Cohort Study (KFACS). J Nutr Health Aging. 2018;22:1275–80.
Granger CV, Dewis LS, Peters NC, Sherwood CC, Barrett JE. Stroke rehabilitation: analysis of repeated Barthel index measures. Arch Phys Med Rehabil. 1979;60:14–7.
Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70.
Kwah LK, Diong J. National institutes of health stroke scale (NIHSS). J Physiother. 2014;60:61.
Nair R, Ferguson H, Stark DL, Lincoln NB. Memory rehabilitation for people with multiple sclerosis. Cochrane Database Syst Rev. 2012;3:CD008754.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17:39.
Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol. 2014;14:120.
Mascha EJ. Alpha, beta, meta: guidelines for assessing power and type I error in meta-analyses. Anesth Analg. 2015;121:1430–3.
Ni J, Wang L, Wang F, Jiang M, Dujuan S. Tranexamic acid for spontaneous intracerebral hemorrhage: a randomized controlled study. Int J Cerebrovasc Dis. 2020;28:5.
Sprigg N, Flaherty K, Appleton JP, Salman RAS, Bereczki D, Beridze M, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391:2107–15.
Liu J, Nie X, Gu H, Zhou Q, Sun H, Tan Y, et al. Tranexamic acid for acute intracerebral haemorrhage growth based on imaging assessment (TRAIGE): a multicentre, randomised, placebo-controlled trial. Stroke Vasc Neurol. 2021;6(2):160–9.
Brenner A, Belli A, Chaudhri R, Coats T, Frimley L, Jamaluddin SF, et al. Understanding the neuroprotective effect of tranexamic acid: an exploratory analysis of the CRASH-3 randomised trial. Crit Care. 2020;24:560.
Brenner A, Arribas M, Cuzick J, Jairath V, Stanworth S, Ker K, et al. Outcome measures in clinical trials of treatments for acute severe haemorrhage. Trials. 2018;19:533.
Huang B, Xu Q, Ye R, Xu J. Influence of tranexamic acid on cerebral hemorrhage: a meta-analysis of randomized controlled trials. Clin Neurol Neurosurg. 2018;171:174–8.
Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–60.
Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9:840–55.
Roach ES, Golomb MR, Adams R, Biller J, Daniels S, Deveber G, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular disease in the young. Stroke. 2008;39:2644–91.
Wesley MC, Pereira LM, Scharp LA, Emani SM, McGowan FX Jr, DiNardo JA. Pharmacokinetics of tranexamic acid in neonates, infants, and children undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2015;122:746–58.
Faraoni D, Goobie SM. The efficacy of antifibrinolytic drugs in children undergoing noncardiac surgery: a systematic review of the literature. Anesth Analg. 2014;118:628–36.
Beno S, Ackery AD, Callum J, Rizoli S. Tranexamic acid in pediatric trauma: why not? Crit Care. 2014;18:313.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The National Key R&D Program of China (No. 2018YFA0108604; No. 2018YFA0108603); 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (2018HXFH008; 2018HXFH010).
Conflict of interest
Xing Wang, Lu Ma, Jinlei Song, and Chao You have indicated they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The authors agreed to share data by reasonable request from a qualified investigator.
Code availability
Not applicable.
Author contributions
XW and CY designed the meta-analysis. XW, JS, and LM searched for relevant studies, selected the studies, and extracted the relevant information. XW and LM synthesized the data. XW wrote the first draft of the paper. All authors revised the manuscript and approved the final version as submitted and agree to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Ma, L., Song, J. et al. Tranexamic Acid for Adult Patients with Spontaneous Intracerebral Hemorrhage: A Systematic Review with Meta-analysis. CNS Drugs 35, 1163–1172 (2021). https://doi.org/10.1007/s40263-021-00865-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00865-2