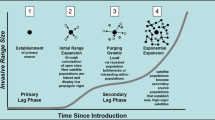
Abstract
An invasive plant species may restrict its spread to only one type of habitat, or, after some time, may continue to spread into a different, often stressful, secondary, habitat. The question of whether evolution is required for an invasive species to spread from one habitat to another is currently hotly debated. In order for local adaptation to occur, genetic variation must be present within invasive populations. In this paper, I focus on the effect of habitat on the maintenance of genetic variation during the lag phase, the phase of population stability prior to expansion. Genetic diversity in invasive plant populations accumulates through multiple introductions, gene flow, mutation, and hybridization, but diversity is maintained by population level processes influencing effective population size (Ne). I show that when the plastic response to the environment results in little variation in reproductive output among individuals, Ne is maximized and genetic variation is maintained. Established models of plant competition show that below-ground competition reduces the variation in reproductive output, whereas competition for light increases variation in reproductive output. The same environments that maintain high Ne also reduce the opportunity for selection and minimize the response to selection, and thus the effects of the environment are synchronized to prevent genetic purges. When the primary invasion habitat supports high Ne, conditions are ripe for local adaptation to a secondary habitat, particularly if the secondary habitat has high opportunity for selection. When the primary invasion habitat supports low Ne, genetic diversity is less likely to be sufficient for local adaptation to secondary habitat to occur.
Similar content being viewed by others
References
Alexander J M, Kueffer C, Daehler C C, et al. 2011. Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proceedings of the National Academy of Sciences, 108(2): 656–661.
Arnold S J, Wade M J. 1984. On the measurement of natural and sexual selection: theory. Evolution, 38(4): 709–719.
Christiansen F B. 1990. Simplified models for viability selection at multiple loci. Theoretical Population Biology, 37: 39–54.
Colautti R I, Ricciardi A, Grigorovich I A, et al. 2004. Is invasion success explained by the enemy release hypothesis? Ecology Letters, 7: 721–733.
Crow J F, Denniston C. 1988. Inbreeding and variance effective population numbers. Evolution, 42(3): 482–495.
Crow J F. 1989. Fitness variation in natural populations. In: Hill W G, Mackay T F C. Evolution and Animal Breeding: Reviews on Molecular and Quantitative Approaches in Honor of Alan Robertson, Wallingford: CAB International, 91–97.
Davis M A, Grime P J, Thompson K. 2000. Fluctuating resources in plant communities: a general theory of invisibility. Journal of Ecology, 88(3): 528–534.
Dietz H, Edwards P J. 2006. Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology, 87(6): 1359–1367.
Dlugosch K M, Parker I M. 2008a. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology, 17: 431–449.
Dlugosch K M, Parker I M. 2008b. Invading populations of an ornamental shrub show rapid life history evolution despite genetic bottlenecks. Ecology Letters, 11: 701–709.
Ellstrand N C. 2009. Evolution of invasiveness in plants following hybridization. Biological Invasions, 11: 1089–1091.
Erfmeier A, Bohnke M, Bruelheide H. 2011. Secondary invasion of Acer negundo: the role of phenotypic responses versus local adaptation. Biological Invasions, 13: 1599–1614.
Espeland E K, Rice K J. 2010. Ecological effects on estimates of effective population size in an annual plant. Biological Conservation, 143: 946–951.
Frank S A, Slatkin M. 1992. Fisher’s fundamental theorem of natural selection. Trends in Ecology and Evolution, 7(3): 92–95.
Frankham R. 1995. Effective population size/adult population size ratios in wildlife: a review. Genetics Research, 66: 95–107.
Goldringer I, Enjalbert J, Raquin A L, et al. 2001. Strong selection in wheat populations during ten generations of dynamic management. Genetics Selection Evolution, 33(S1): 441–463.
Goldringer I, Bataillon T. 2004. On the distribution of temporal variations in allele frequency: consequences for the estimation of effective population size and detection of loci undergoing selection. Genetics, 168: 563–568.
Haider S, Kueffer C, Edwards P J, et al. 2012. Genetically based differentiation in growth of multiple non-native plant species along a steep environmental gradient. Population Ecology, 170: 89–99.
Hartl D L, Clark A G. 2007. Principles of Population Genetics. Sunderland: Sinauer Associates.
Hedrick P. 2005. Large variance in reproductive success and the Ne/N ratio. Evolution, 59(7): 1596–1599.
Hereford J. 2010. Does selfing or outcrossing promote local adaptation? American Journal of Botany, 97(2): 298–302.
Hufbauer R A, Facon B, Ravigne V, et al. 2012. Anthropogenically induced adaptation to invade (AIAI): contemporary adaptation to human-altered habitats within the native range can promote invasions. Evolutionary Applications, doi: 10.1111/j.1752-4571.2011.00211.x.
Huttanus T D, Mack R N, Novak S J. 2011. Propagule pressure and introduction pathways of Bromus tectorum (cheatgrass; Poaceae) in the central United States. International Journal of Plant Sciences, 172(6): 783–794.
Jones E I, Gomulkiewicz R. 2012. Biotic interactions, rapid evolution, and the establishment of introduced species. The American Naturalist, 179(2): 28–36.
Jordan N R, Larson D L, Huerd S C. 2008. Soil modification by invasive plants: effects on native and invasive species of mixed-grass prairies. Biological Invasions, 10: 177–190.
Kalinowski S T, Waples R S. 2002. Relationship of effective to census size in fluctuating populations. Conservation Biology, 16(1): 129–136.
Leger E A, Espeland E K. 2010. Coevolution between native and invasive plant competitors: implications for invasive species management. Evolutionary Applications, 3: 169–178.
Leimu R, Fischer M. 2008. A meta-analysis of local adaptation in plants. PLoS One, 3(12): e4010.
Lewontin R C, Kojima K. 1960. The evolutionary dynamics of complex polymorphisms. Evolution, 14(4): 458–472.
Lockwood J L, Cassey P, Blackburn T. 2005. The role of propagule pressure in explaining species invasions. Trends in Ecology and Evolution, 20: 223–228.
Lynch M, Conery J, Burger R. 1995. Mutation accumulation and the extinction of small populations. The American Naturalist, 146(4): 489–518.
Martina J P, von Ende C N. 2012. Highly plastic response in morphological and physiological traits to light, soil-N and moisture in the model invasive plant, Phalaris arundinacea. Environmental and Experimental Botany, 82: 43–53.
Meimberg H, Milan N F, Karatassiou M, et al. 2010. Patterns of introduction and adaptation during the invasion of Aegilops triuncialis (Poaceae) into Californian serpentine soils. Molecular Ecology, 19: 5308–5319.
Monaco T A, Johnson D A, Creech J E. 2005. Morphological and physiological responses of the invasive weed Isatis tinctoria to contrasting light, soil-nitrogen and water. Weed Research, 45: 460–466.
Novak S J, Rausch J H. 2009. Use of field surveys, distributional data and genetic analyses to monitor alien species: Taeniatherum caput-medusae as an example of the approach. Neobiota, 8: 169–182.
Nunney L. 1995. Measuring the ratio of effective population size to adult numbers using genetic and ecological data. Evolution, 49(2): 389–392
Pysek P, Vojtech J, Pergl J, et al. 2011. Colonization of high altitudes by alien plants over the last two centuries. Proceedings of the National Academy of Sciences, 108(2): 439–440.
Reed D H. 2005. Relationship between population size and fitness. Conservation Biology, 19: 563–568.
Richards C L, Schrey A W, Pigliucci M. 2012. Invasion of diverse habitats by few Japanese knotweed genotypes is correlated with epigenetic differentiation. Ecology Letters, 15: 1016–1025.
Schierenbeck K A, Ellstrand N C. 2009. Hybridization and the evolution of invasiveness in plants and other organisms. Biological Invasions, 11: 1093–1105.
Schwinning S. 1996. Decomposition analysis of competitive symmetry and size structure dynamics. Annals of Botany, 77: 47–57.
Siol M, Bonnin I, Oliveri I, et al. 2007. Effective population size associated with self-fertilization: lessons from temporal changes in allele frequencies in the selfing annual Medicago trunculata. Journal of Evolutionary Biology, 20: 2349–2360.
Sultan S E, Horgan-Kobelski T, Nichols L M, et al. 2012. A resurrection study reveals rapid adaptive evolution within populations of an invasive plant. Evolutionary Applications, doi:10.1111/j.1752-4571.2012.00287.x.
Van Kleunen M, Fischer M, Schmid B. 2001. Effects of intraspecific competition on size variation and reproductive allocation in a clonal plant. Oikos, 94: 515–524.
Van Kleunen M, Fischer M, Schmid B. 2005. Three generations under low versus high neighborhood density affect the life history of a clonal plant through differential selection and genetic drift. Oikos, 108: 573–581.
Weiner, J, Thomas S C. 1986. Size variability and competition in plant monocultures. Oikos, 47: 211–222.
Willi Y, van Buskirk J, Hoffmann A A. 2006. Limits to the adaptive potential of small populations. Annual Review of Ecology Evolution and Systematics, 37: 433–458.
Willi Y, Hoffmann A A. 2009. Demographic factors and genetic variation influence population persistence under environmental change. Journal of Evolutionary Biology, 22: 124–133.
Wilson J B, Levin D A. 1986. Some genetic consequences of skewed fecundity distributions in plants. Theoretical and Applied Genetics, 73: 113–121.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Espeland, E.K. Predicting the dynamics of local adaptation in invasive species. J. Arid Land 5, 268–274 (2013). https://doi.org/10.1007/s40333-013-0163-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40333-013-0163-1