Abstract
Purpose
To investigate the glucocorticoid-induced impairments of muscle mass and structure in patients presenting different stages of steroid myopathy progression.
Methods
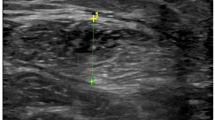
Thirty-three patients (28 women) affected by active (N = 20) and remitted (N = 13) Cushing’s disease were recruited and the following variables were assessed: walking speed, handgrip strength, total body and appendicular muscle mass by bioelectrical impedance analysis (BIA), thickness and echo intensity of lower limb muscles by ultrasonography.
Results
The two groups of patients showed comparable values of both handgrip strength [median (interquartile range) values: active disease: 27.4 (7.5) kg vs. remitted disease: 26.4 (9.4) kg; P = 0.58] and walking speed [active disease: 1.0 (0.2) m/s vs. remitted disease: 1.1 (0.3) m/s; P = 0.43]. Also, the thickness of the four muscles and all BIA-derived sarcopenic indices were comparable (P > 0.05 for all comparisons) between the two groups. On the contrary, the echo intensity of vastus lateralis, tibialis anterior (lower portion), and medial gastrocnemius was significantly (P < 0.05 for all comparisons) higher in patients with active disease compared to patients with remitted disease. Finally, significant negative correlations were found in the whole group of patients between muscle echo intensity and muscle function assessments.
Conclusions
We provided preliminary evidence that the ultrasound-derived measurements of muscle thickness and echo intensity can be useful to detect and track the changes of muscle mass and structure in patients with steroid myopathy and we suggest that the combined assessment of muscle mass, strength, and performance should be systematically applied in the routine examination of steroid myopathy patients.




Similar content being viewed by others
References
Minetto MA, Lanfranco F, Motta G, Allasia S, Arvat E, D’Antona G (2011) Steroid myopathy: some unresolved issues. J Endocrinol Investig 34:370–375
Pereira RM, Freire de Carvalho J (2011) Glucocorticoid-induced myopathy. Jt Bone Spine 78:41–44
Schakman O, Kalista S, Barbé C, Loumaye A, Thissen JP (2013) Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 45:2163–2172
Minetto MA, D’Angelo V, Arvat E, Kesari S (2018) Diagnostic work-up in steroid myopathy. Endocrine 60:219–223
Minetto MA, Rainoldi A, Jabre JF (2007) The clinical use of macro and surface electromyography in diagnosis and follow-up of endocrine and drug-induced myopathies. J Endocrinol Investig 30:791–796
Minetto MA, Botter A, Lanfranco F, Baldi M, Ghigo E, Arvat E (2010) Muscle fiber conduction slowing and decreased levels of circulating muscle proteins after short-term dexamethasone administration in healthy subjects. J Clin Endocrinol Metab 95:1663–1671
Minetto MA, Lanfranco F, Botter A, Motta G, Mengozzi G, Giordano R, Picu A, Ghigo E, Arvat E (2011) Do muscle fiber conduction slowing and decreased levels of circulating muscle proteins represent sensitive markers of steroid myopathy? A pilot study in Cushing’s disease. Eur J Endocrinol 164:985–993
Blijham PJ, ter Laak HJ, Schelhaas HJ, van Engelen BG, Stegeman DF, Zwarts MJ (2006) Relation between muscle fiber conduction velocity and fiber size in neuromuscular disorders. J Appl Physiol (1985) 100:1837–1841
Minetto MA, Qaisar R, Agoni V, Motta G, Longa E, Miotti D, Pellegrino MA, Bottinelli R (2015) Quantitative and qualitative adaptations of muscle fibers to glucocorticoids. Muscle Nerve 52:631–639
Farina D, Merletti R, Enoka RM (2004) The extraction of neural strategies from the surface EMG. J Appl Physiol (1985) 96:1486–1495
Farina D, Falla D (2008) Effect of muscle-fiber velocity recovery function on motor unit action potential properties in voluntary contractions. Muscle Nerve 37:650–658
Kamavuako EN, Farina D (2010) Time-dependent effects of pre-conditioning activation on muscle fiber conduction velocity and twitch torque. Muscle Nerve 42:547–555
Reeves ND, Maganaris CN, Narici MV (2004) Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol 91:116–118
Arts IM, Pillen S, Schelhaas HJ, Overeem S, Zwarts MJ (2010) Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve 41:32–41
Noorkoiv M, Nosaka K, Blazevich AJ (2010) Assessment of quadriceps muscle cross-sectional area by ultrasound extended-field-of-view imaging. Eur J Appl Physiol 109:631–639
Narici MV, Binzoni T, Hiltbrand E, Fasel J, Terrier F, Cerretelli P (1996) In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol 496:287–297
Kwah LK, Pinto RZ, Diong J, Herbert RD (2013) Reliability and validity of ultrasound measurements of muscle fascicle length and pennation in humans: a systematic review. J Appl Physiol (1985) 114:761–769
Pillen S, Arts IM, Zwarts MJ (2008) Muscle ultrasound in neuromuscular disorders. Muscle Nerve 37:679–693
Seymour JM, Ward K, Sidhu PS, Puthucheary Z, Steier J, Jolley CJ, Rafferty G, Polkey MI, Moxham J (2009) Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax 64:418–423
Atkinson RA, Srinivas-Shankar U, Roberts SA, Connolly MJ, Adams JE, Oldham JA, Wu FC, Seynnes OR, Stewart CE, Maganaris CN, Narici MV (2010) Effects of testosterone on skeletal muscle architecture in intermediate-frail and frail elderly men. J Gerontol A Biol Sci Med Sci 65:1215–1219
Nijholt W, Scafoglieri A, Jager-Wittenaar H, Hobbelen JSM, van der Schans CP (2017) The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle 8:702–712
Pillen S, Tak RO, Zwarts MJ, Lammens MM, Verrijp KN, Arts IM, van der Laak JA, Hoogerbrugge PM, van Engelen BG, Verrips A (2009) Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol 35:443–446
Hu CF, Chen CP, Tsai WC, Hu LL, Hsu CC, Tseng ST, Shau YW (2012) Quantification of skeletal muscle fibrosis at different healing stages using sonography: a morphologic and histologic study in an animal model. J Ultrasound Med 31:43–88
Arts IM, Schelhaas HJ, Verrijp KC, Zwarts MJ, Overeem S, van der Laak JA, Lammens MM, Pillen S (2012) Intramuscular fibrous tissue determines muscle echo intensity in amyotrophic lateral sclerosis. Muscle Nerve 45:449–450
Caresio C, Molinari F, Emanuel G, Minetto MA (2015) Muscle echo intensity: reliability and conditioning factors. Clin Physiol Funct Imaging 35:393–403
Garrapa GG, Pantanetti P, Arnaldi G, Mantero F, Faloia E (2001) Body composition and metabolic features in women with adrenal incidentaloma or Cushing’s syndrome. J Clin Endocrinol Metab 86:5301–5306
Resmini E, Farkas C, Murillo B, Barahona MJ, Santos A, Martínez-Momblán MA, Roig O, Ybarra J, Geli C, Webb SM (2010) Body composition after endogenous (Cushing’s syndrome) and exogenous (rheumatoid arthritis) exposure to glucocorticoids. Horm Metab Res 42:613–618
London E, Lodish M, Keil M, Lyssikatos C, de la Luz Sierra M, Nesterova M, Stratakis CA (2014) Not all glucocorticoid-induced obesity is the same: differences in adiposity among various diagnostic groups of Cushing syndrome. Horm Metab Res 46:897–903
Minetto MA, Caresio C, D’Angelo V, Lanfranco F, Ghizzoni L, Roatta S, Arvat E, Kesari S (2018) Diagnostic evaluation in steroid-induced myopathy: case report suggesting clinical utility of quantitative muscle ultrasonography. Endocr Res 12:1–11
Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos G, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M (2003) Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 88:5593–5602
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM (2008) The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93:1526–1540
Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A (2016) Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol 4:611–629
Berr CM, Stieg MR, Deutschbein T, Quinkler M, Schmidmaier R, Osswald A, Reisch N, Ritzel K, Dimopoulou C, Fazel J, Hahner S, Stalla GK, Beuschlein F, Reincke M (2017) Persistence of myopathy in Cushing’s syndrome: evaluation of the German Cushing’s Registry. Eur J Endocrinol 176:737–746
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M (2010) European Working Group on Sarcopenia in Older People, Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423
Janssen I, Heymsfield SB, Baumgartner RN, Ross R (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985) 89:465–471
Sergi G, De Rui M, Veronese N, Bolzetta F, Berton L, Carraro S, Bano G, Coin A, Manzato E, Perissinotto E (2015) Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin Nutr 34:667–673
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896
Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R (2004) Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 159:413–421
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TT, Kenny AM, Fragala MS, Harris TB, Kiel DP, Guralnik JM, Ferrucci L, Kritchevsky SB, Vassileva MT, Studenski SA, Alley DE (2014) Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 69:567–575
Molinari F, Caresio C, Acharya UR, Mookiah MR, Minetto MA (2015) Advances in quantitative muscle ultrasonography using texture analysis of ultrasound images. Ultrasound Med Biol 41:2520–2532
Minetto MA, Caresio C, Menapace T, Hajdarevic A, Marchini A, Molinari F, Maffiuletti NA (2016) Ultrasound-based detection of low muscle mass for diagnosis of sarcopenia in older adults. PM&R 8:453–462
Caresio C, Salvi M, Molinari F, Meiburger KM, Minetto MA (2017) Fully automated muscle ultrasound analysis (MUSA): robust and accurate muscle thickness measurement. Ultrasound Med Biol 43:195–205
Geer EB, Shen W, Strohmayer E, Post KD, Freda PU (2012) Body composition and cardiovascular risk markers after remission of Cushing’s disease: a prospective study using whole-body MRI. J Clin Endocrinol Metab 97:1702–1711
Lopez P, Wilhelm EN, Rech A, Minozzo F, Radaelli R, Pinto RS (2017) Echo intensity independently predicts functionality in sedentary older men. Muscle Nerve 55:9–15
Mirón Mombiela R, Facal de Castro F, Moreno P, Borras C (2017) Ultrasonic echo intensity as a new noninvasive in vivo biomarker of frailty. J Am Geriatr Soc 65:2685–2690
Pillen S, van Dijk JP, Weijers G, Raijmann W, de Korte CL, Zwarts MJ (2009) Quantitative gray-scale analysis in skeletal muscle ultrasound: a comparison study of two ultrasound devices. Muscle Nerve 39:781–786
Seene T, Kaasik P (2016) Role of myofibrillar protein catabolism in development of glucocorticoid myopathy: aging and functional activity aspects. Metabolites 6:E15
Maffiuletti NA (2010) Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol 110:223–234
Lukaski HC, Bolonchuk WW, Hall CB, Siders WA (1986) Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol (1985) 60:1327–1332
Moon JR (2013) Body composition in athletes and sports nutrition: an examination of the bioimpedance analysis technique. Eur J Clin Nutr 67:S54–S59
Drey M, Berr CM, Reincke M, Fazel J, Seissler J, Schopohl J, Bidlingmaier M, Zopp S, Reisch N, Beuschlein F, Osswald A, Schmidmaier R (2017) Cushing’s syndrome: a model for sarcopenic obesity. Endocrine 57:481–485
Kemink SA, Frijns JT, Hermus AR, Pieters GF, Smals AG, van Marken Lichtenbelt WD (1999) Body composition determined by six different methods in women bilaterally adrenalectomized for treatment of Cushing’s disease. J Clin Endocrinol Metab 84:3991–3999
Pirlich M, Biering H, Gerl H, Ventz M, Schmidt B, Ertl S, Lochs H (2002) Loss of body cell mass in Cushing’s syndrome: effect of treatment. J Clin Endocrinol Metab 87:1078–1084
Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL (2011) Hand grip strength: age and gender stratified normative data in a population-based study. BMC Res Notes 4:127
Baudry S, Motta G, Botter A, Duchateau J, Minetto MA (2018) Neural correlates to the increase in maximal force after dexamethasone administration. Med Sci Sports Exerc 50:218–224
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclosure.
Ethical approval
The study was approved by the local ethics committee. The study conformed with the guidelines in the Declaration of Helsinki and was approved by the local ethics committee.
Informed consent
All patients received a detailed explanation of the study and gave written informed consent prior to participation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Minetto, M.A., Caresio, C., Salvi, M. et al. Ultrasound-based detection of glucocorticoid-induced impairments of muscle mass and structure in Cushing’s disease. J Endocrinol Invest 42, 757–768 (2019). https://doi.org/10.1007/s40618-018-0979-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0979-9