Abstract
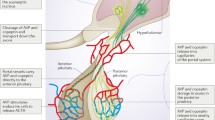
Copeptin
Copeptin is secreted in equimolar amount to Arginine Vasopressin (AVP) but can easily be measured with a sandwich immunoassay. Both peptides, copeptin and AVP, show a high correlation. Accordingly, copeptin mirrors the amount of AVP in the circulation and its measurement provides an attractive marker in the differential diagnosis of diabetes insipidus.
The polyuria polydipsia syndrome
Diabetes insipidus—either central or nephrogenic—has to be differentiated from primary polydipsia. Differentiation is crucial since wrong treatment can have deleterious consequences. Since many decades, the “gold standard” for differential diagnosis has been the classical water deprivation test, which has several limitations leading to an overall limited diagnostic accuracy. In addition, the test has a long duration of 17 hours and is cumbersome for patients. Clinical signs and symptoms as well as MRI characteristics overlap between patients with diabetes insipidus and primary polydipsia. Direct measurement of AVP upon osmotic stimulation was first shown to overcome these limitations, but failed to enter clinical practice mainly due to technical limitations of the AVP assay.
Copeptin as diagnostic tool in the polyuria polydipsia syndrome
We have recently shown that copeptin, without prior water deprivation, identifies patients with nephrogenic diabetes insipidus. On the other hand, for the more difficult differentiation between central diabetes insipidus and primary polydipsia, a copeptin level of 4.9 pmol/L stimulated with hypertonic saline infusion differentiates between these two entities with a high diagnostic accuracy, and is superior to the water deprivation test. It is important to note that close sodium monitoring during the hypertonic saline test is a prerequisite.
Conclusion
Therefore, we propose that copeptin upon hypertonic saline infusion should become the new standard test in the differential diagnosis of diabetes insipidus.
Similar content being viewed by others
References
Robertson GL (1995) Diabetes insipidus. Endocrinol Metab Clin N Am 24(3):549–572
Holwerda DA (1972) A glycopeptide from the posterior lobe of pig pituitaries I Isolation and characterization. Eur J Biochem FEBS 28(3):334–339
Levy B, Chauvet MT, Chauvet J, Acher R (1986) Ontogeny of bovine neurohypophysial hormone precursors. II. Foetal copeptin, the third domain of the vasopressin precursor. Int J Peptide Protein Res 27(3):320–324
Land H, Schutz G, Schmale H, Richter D (1982) Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature 295(5847):299–303
Morgenthaler NG, Struck J, Alonso C, Bergmann A (2006) Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52(1):112–119
Nathanson MH, Moyer MS, Burgstahler AD, O’Carroll AM, Brownstein MJ, Lolait SJ (1992) Mechanisms of subcellular cytosolic Ca2+ signaling evoked by stimulation of the vasopressin V1a receptor. J Biol Chem 267(32):23282–23289
Baylis PH (1987) Osmoregulation and control of vasopressin secretion in healthy humans. Am J Physiol 253(5 Pt 2):R671–R678
Christ-Crain M, Fenske W (2016) Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat Rev Endocrinol 12(3):168–176
Nagy G, Mulchahey JJ, Smyth DG, Neill JD (1988) The glycopeptide moiety of vasopressin-neurophysin precursor is neurohypophysial prolactin releasing factor. Biochem Biophys Res Commun 151(1):524–529
Hyde JF, North WG, Ben-Jonathan N (1989) The vasopressin-associated glycopeptide is not a prolactin-releasing factor: studies with lactating Brattleboro rats. Endocrinology 125(1):35–40
Barat C, Simpson L, Breslow E (2004) Properties of human vasopressin precursor constructs: inefficient monomer folding in the absence of copeptin as a potential contributor to diabetes insipidus. Biochemistry 43(25):8191–8203
Parodi AJ (2000) Protein glucosylation and its role in protein folding. Annu Rev Biochem 69:69–93
Birk J, Friberg MA, Prescianotto-Baschong C, Spiess M, Rutishauser J (2009) Dominant pro-vasopressin mutants that cause diabetes insipidus form disulfide-linked fibrillar aggregates in the endoplasmic reticulum. J Cell Sci 122(Pt 21):3994–4002
Fenske W, Refardt J, Chifu I et al (2018) A copeptin-based approach in the diagnosis of diabetes insipidus. N Engl J Med 379(5):428–439
Timper K, Fenske W, Kuhn F et al (2015) Diagnostic accuracy of copeptin in the differential diagnosis of the polyuria-polydipsia syndrome: a prospective multicenter study. J Clin Endocrinol Metab 100(6):2268–2274
Balanescu S, Kopp P, Gaskill MB, Morgenthaler NG, Schindler C, Rutishauser J (2011) Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar States. J Clin Endocrinol Metab 96(4):1046–1052
Fenske WK, Schnyder I, Koch G et al (2018) Release and decay kinetics of copeptin vs AVP in response to osmotic alterations in healthy volunteers. J Clin Endocrinol Metab 103(2):505–513
Szinnai G, Morgenthaler NG, Berneis K et al (2007) Changes in plasma copeptin, the c-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab 92(10):3973–3978
Morgenthaler NG, Muller B, Struck J, Bergmann A, Redl H, Christ-Crain M (2007) Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock. 28(2):219–226
Katan M, Fluri F, Morgenthaler NG et al (2009) Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol 66(6):799–808
Reichlin T, Hochholzer W, Stelzig C et al (2009) Incremental value of copeptin for rapid rule out of acute myocardial infarction. J Am Coll Cardiol 54(1):60–68
Katan M, Christ-Crain M (2010) The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 140:w13101
Katan M, Morgenthaler N, Widmer I et al (2008) Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett 29(3):341–346
Siegenthaler J, Walti C, Urwyler SA, Schuetz P, Christ-Crain M (2014) Copeptin concentrations during psychological stress: the PsyCo study. Eur J Endocrinol 171(6):737–742
Urwyler SA, Schuetz P, Sailer C, Christ-Crain M (2015) Copeptin as a stress marker prior and after a written examination—the CoEXAM study. Stress 18(1):134–137
Maeder MT, Staub D, Brutsche MH et al (2010) Copeptin response to clinical maximal exercise tests. Clin Chem 56(4):674–676
Hew-Butler T, Hoffman MD, Stuempfle KJ, Rogers IR, Morgenthaler NG, Verbalis JG (2011) Changes in copeptin and bioactive vasopressin in runners with and without hyponatremia. Clin J Sport Med 21(3):211–217
Robertson GL (2001) Antidiuretic hormone. Normal and disordered function. Endocrinol Metab Clin N Am 30(3):671–694 (vii)
Nakajima A, Lu Y, Kawano H, Horie S, Muto S (2015) Association of arginine vasopressin surrogate marker urinary copeptin with severity of autosomal dominant polycystic kidney disease (ADPKD). Clin Exp Nephrol 19(6):1199–1205
Roussel R, Fezeu L, Marre M et al (2014) Comparison between copeptin and vasopressin in a population from the community and in people with chronic kidney disease. J Clin Endocrinol Metab 99(12):4656–4663
Bhandari SS, Loke I, Davies JE, Squire IB, Struck J, Ng LL (2009) Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci (Lond) 116(3):257–263
Darzy KH, Dixit KC, Shalet SM, Morgenthaler NG, Brabant G (2010) Circadian secretion pattern of copeptin, the C-terminal vasopressin precursor fragment. Clin Chem 56(7):1190–1191
Beglinger S, Drewe J, Christ-Crain M (2016) The circadian rhythm of copeptin, the C-terminal portion of Arginin Vasopressin. In: Poster presentation, SGED Congress Nov. 17–18, 2016, Bern, Switzerland
Puder JJ, Blum CA, Mueller B, De Geyter C, Dye L, Keller U (2006) Menstrual cycle symptoms are associated with changes in low-grade inflammation. Eur J Clin Investig 36(1):58–64
Walti C, Siegenthaler J, Christ-Crain M (2014) Copeptin levels are independent of ingested nutrient type after standardised meal administration—the CoMEAL study. Biomarkers 19(7):557–562
Robertson GL (1976) The regulation of vasopressin function in health and disease. Recent Prog Horm Res 33:333–385
Miller M, Dalakos T, Moses AM, Fellerman H, Streeten DH (1970) Recognition of partial defects in antidiuretic hormone secretion. Ann Intern Med 73(5):721–729
Bockenhauer D, Bichet DG (2015) Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol 11(10):576–588
Nemergut EC, Zuo Z, Jane JA Jr, Laws ER Jr (2005) Predictors of diabetes insipidus after transsphenoidal surgery: a review of 881 patients. J Neurosurg 103(3):448–454
Babey M, Kopp P, Robertson GL (2011) Familial forms of diabetes insipidus: clinical and molecular characteristics. Nat Rev Endocrinol 7(12):701–714
Thompson CJ, Baylis PH (1987) Thirst in diabetes insipidus: clinical relevance of quantitative assessment. Q J Med 65(246):853–862
Epstein FH, Kleeman CR, Hendrikx A (1957) The influence of bodily hydration on the renal concentrating process. J Clin Investig 36(5):629–634
Fenske W, Allolio B (2012) Clinical review: current state and future perspectives in the diagnosis of diabetes insipidus: a clinical review. J Clin Endocrinol Metab 97(10):3426–3437
Carter AC, Robbins J (1947) The use of hypertonic saline infusions in the differential diagnosis of diabetes insipidus and psychogenic polydipsia. J Clin Endocrinol Metab 7(11):753–766
Barlow ED, De Wardener HE (1959) Compulsive water drinking. Q J Med 28(110):235–258
Fenske W, Quinkler M, Lorenz D et al (2011) Copeptin in the differential diagnosis of the polydipsia-polyuria syndrome—revisiting the direct and indirect water deprivation tests. J Clin Endocrinol Metab 96(5):1506–1515
Palevsky PM, Bhagrath R, Greenberg A (1996) Hypernatremia in hospitalized patients. Ann Intern Med 124(2):197–203
Fujisawa I, Nishimura K, Asato R et al (1987) Posterior lobe of the pituitary in diabetes insipidus: MR findings. J Comput Assist Tomogr 11(2):221–225
Arslan A, Karaarslan E, Dincer A (1999) High intensity signal of the posterior pituitary. A study with horizontal direction of frequency-encoding and fat suppression MR techniques. Acta Radiol 40(2):142–145
Moses AM, Clayton B, Hochhauser L (1992) Use of T1-weighted MR imaging to differentiate between primary polydipsia and central diabetes insipidus. AJNR Am J Neuroradiol 13(5):1273–1277
Cote M, Salzman KL, Sorour M, Couldwell WT (2014) Normal dimensions of the posterior pituitary bright spot on magnetic resonance imaging. J Neurosurg 120(2):357–362
Hannon MJ, Orr C, Moran C et al (2012) Anterior hypopituitarism is rare and autoimmune disease is common in adults with idiopathic central diabetes insipidus. Clin Endocrinol (Oxf) 76(5):725–728
Zerbe RL, Robertson GL (1981) A comparison of plasma vasopressin measurements with a standard indirect test in the differential diagnosis of polyuria. N Engl J Med 305(26):1539–1546
Baylis PH, Gaskill MB, Robertson GL (1981) Vasopressin secretion in primary polydipsia and cranial diabetes insipidus. Q J Med 50(199):345–358
Milles JJ, Spruce B, Baylis PH (1983) A comparison of diagnostic methods to differentiate diabetes insipidus from primary polyuria: a review of 21 patients. Acta Endocrinol (Copenh) 104(4):410–416
Kluge M, Riedl S, Erhart-Hofmann B, Hartmann J, Waldhauser F (1999) Improved extraction procedure and RIA for determination of arginine 8-vasopressin in plasma: role of premeasurement sample treatment and reference values in children. Clin Chem 45(1):98–103
Fenske WK, Schnyder I, Koch G et al (2017) Release and decay kinetics of copeptin versus AVP in response to osmotic alterations in healthy volunteers. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2017-01891
Katan M, Morgenthaler NG, Dixit KC et al (2007) Anterior and posterior pituitary function testing with simultaneous insulin tolerance test and a novel copeptin assay. J Clin Endocrinol Metab 92(7):2640–2643
Robertson GL, Shelton RL, Athar S (1976) The osmoregulation of vasopressin. Kidney Int 10(1):25–37
Rosen CJ, Ingelfinger JR (2018) A reliable diagnostic test for hypotonic polyuria. N Engl J Med 379(5):483–484
Gubbi S, Hannah-Shmouni F, Koch CA, Verbalis JG (2000) Diagnostic Testing for Diabetes Insipidus. In: Feingold KR, Anawalt B, Boyce A et al (eds) Endotext. MDText.com, Inc., South Dartmouth
Funding
M. Christ-Crain was supported by a grant from the Swiss National Science Foundation (SNF-162608) and the University Hospital Basel, Switzerland.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
MCC and WF received speaker honoraria from Thermofisher AG, the manufacturer of the Copeptin assay.
Ethical approval
All procedures involving human participants were in accordance with ethical standards of the institutional and/or national research committee with the Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individuals included in one of the cited studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Christ-Crain, M., Fenske, W.K. Copeptin in the differential diagnosis of hypotonic polyuria. J Endocrinol Invest 43, 21–30 (2020). https://doi.org/10.1007/s40618-019-01087-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01087-6