Abstract
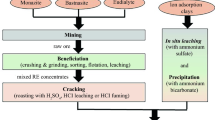
The present study aims to improve the impurity removal step in the rare-earth elements (REE) processing. The fast-growing demand for REE in recent years has attracted special attention to their extraction process. Most of the operating cost associated with the extraction of REE is concentrated in the hydrometallurgical processing of the ores or concentrates. The purity of the final REE product is a valuable key factor to the market. Therefore, impurity removal is an important step in the REE process circuits. This study focuses on improving the neutralization process of sulfate-based REE pregnant leach solutions (PLS), more specifically the Fe and Al removal step. Despite some studies, the mechanism of REE loss during neutralization and impurity removal is still not well understood. Different reagents such as lime, magnesium hydroxide, and sodium hydroxide were investigated in batch experiments. Tests with NaOH presented the minimum REE loss: 17.6% Ce, 20.9% Nd, 24.3% Tb, and 28.8% Er. Tests with Ca(OH)2 led to highest REE loss values: 33.7% Ce, 42.7% Nd, 42.9% Tb, and 51.6% Er. However, calcium-based reagents offer the greatest economic benefit, owing to their low cost. Therefore, further tests were carried out with Ca(OH)2 addition to PLS with varying Fe and Al concentrations. REE loss of 16.3% Ce, 17.6% Nd, 5.8% Tb, and 4.5% Er were registered when 4.0 g/L Fe and 4.0 g/L Al were available in the solution, and the pH was increased from 1.0 to 3.2, which was the largest REE loss. The lowest REE loss (4.0% Ce, 4.3% Nd, 0.7% Tb, and 0.6% Er) was observed when the PLS contained 0.5 g/L Fe and no Al. A two-step impurity removal process was proposed based on the results of this study to remove Fe and Al from the REE PLS. Gypsum–Al–Fe residues produced from the neutralization steps were further treated in an acid leaching step to recover above 80% of the lost REE and improve the efficiency of the process.
Graphical Abstract













Similar content being viewed by others
References
Teixeira LAV, Silva RG (2015) System and process for selective rare earth extraction with sulphur recovery. Patent US 2015/0329940 A1
Simandl GJ (2014) Geology and market-dependent significance of rare earth element resources. Miner Deposita 49:889–904
Simandl GJ (2012) Geology and economic significance of current and future rare earth element sources. Conference of metallurgists-rare earth elements. pp 15–30
Verbaan N, Bradley K, Brown J, Mackie S (2015) A review of hydrometallurgical flowsheets considered in current REE projects. British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Paper 2015-3 pp 147–162
Sadri F, Nazari AM, Ghahreman A (2017) A review on the cracking, baking and leaching processes of rare earth element concentrates. J Rare Earths 35:739–752. https://doi.org/10.1016/S1002-0721(17)60971-2
Keller PC, Anderson DCG (2018) The production of critical materials as by products. AMMS 2:1–14
Judge WD, Azimi G (2020) Recent progress in impurity removal during rare earth element processing: a review. Hydrometallurgy 196:105435. https://doi.org/10.1016/j.hydromet.2020.105435
Jyothi RK, Thenepalli T, Ahn JW et al (2020) Review of rare earth elements recovery from secondary resources for clean energy technologies: grand opportunities to create wealth from waste. J Clean Prod 267:122048. https://doi.org/10.1016/j.jclepro.2020.122048
Mouna HM, Baral SS (2019) A bio-hydrometallurgical approach towards leaching of lanthanum from the spent fluid catalytic cracking catalyst using Aspergillus niger. Hydrometallurgy 184:175–182. https://doi.org/10.1016/j.hydromet.2019.01.007
Muddanna MH, Baral SS (2019) A comparative study of the extraction of metals from the spent fluid catalytic cracking catalyst using chemical leaching and bioleaching by Aspergillus niger. J Environ Chem Eng 7:103335. https://doi.org/10.1016/j.jece.2019.103335
Muddanna MH, Baral SS (2019) Leaching of nickel and vanadium from the spent fluid catalytic cracking catalyst by reconnoitering the potential of Aspergillus niger associating with chemical leaching. J Environ Chem Eng 7:103025. https://doi.org/10.1016/j.jece.2019.103025
Flett DS (1992) Solution purification. Hydrometallurgy 30:327–344. https://doi.org/10.1016/0304-386X(92)90092-E
Ruan C, Jungming X, Yongjun Z (1995) Recovering REE from leaching liquor of rare earth ore by extraction. Trans Nonferrous Metals Soc 5:4
Silva RG, Morais CA, Oliveira ÉD (2020) Evaluation of different neutralization reagents in the selective removal of impurities in rare earth sulfuric liquor. Mining Metall Explor 37:65–78. https://doi.org/10.1007/s42461-019-00139-y
Silva RG, Morais CA, Oliveira ÉD (2019) Selective precipitation of rare earth from non-purified and purified sulfate liquors using sodium sulfate and disodium hydrogen phosphate. Miner Eng 134:402–416. https://doi.org/10.1016/j.mineng.2019.02.028
da Silva RG, de Morais CA, Teixeira LV, de Oliveira ÉD (2018) Selective removal of impurities from rare earth sulphuric liquor using different reagents. Miner Eng 127:238–246. https://doi.org/10.1016/j.mineng.2018.08.007
Önal MAR, Borra CR, Guo M et al (2017) Hydrometallurgical recycling of NdFeB magnets: complete leaching, iron removal and electrolysis. J Rare Earths 35:574–584. https://doi.org/10.1016/S1002-0721(17)60950-5
Kim R, Cho H, Han KN et al (2016) Optimization of acid leaching of rare-earth elements from Mongolian apatite-based ore. Minerals 6:63. https://doi.org/10.3390/min6030063
Sadri F, Kim R, Yang Z, Ghahreman A (2018) The effect of calcium sulfate crystallization and the crystal modification on aqueous REE stability in Ca saturated REE-Ca-SO4-H2O systems. Hydrometallurgy 182:82–96. https://doi.org/10.1016/j.hydromet.2018.10.010
Sadri F, Kim R, Ghahreman A (2019) Substitution of calcium with Ce, Nd, Er, and Tb in the structure of microcrystals of calcium sulfates with controlled hydration water: a proposed mechanism. Cryst Growth Des 19:2621–2631. https://doi.org/10.1021/acs.cgd.8b01744
Kim E, Osseo-Asare K (2012) Aqueous stability of thorium and rare earth metals in monazite hydrometallurgy: Eh–pH diagrams for the systems Th-, Ce-, La-, Nd- (PO4)–(SO4)–H2O at 25 °C. Hydrometallurgy 113–114:67–78. https://doi.org/10.1016/j.hydromet.2011.12.007
Tunsu C, Menard Y, Eriksen DØ et al (2019) Recovery of critical materials from mine tailings: a comparative study of the solvent extraction of rare earths using acidic, solvating and mixed extractant systems. J Clean Prod 218:425–437. https://doi.org/10.1016/j.jclepro.2019.01.312
Akcil A, Koldas S (2006) Acid mine drainage (AMD): causes, treatment and case studies. J Clean Prod 14:1139–1145. https://doi.org/10.1016/j.jclepro.2004.09.006
Dutrizac JE (1983) Jarosite-type compounds and their application in the metallurgical industry. Mineral Sciences Laboratories (CANMET), Society of AIME, New York
Fernando WAM, Ilankoon IMSK, Syed TH, Yellishetty M (2018) Challenges and opportunities in the removal of sulphate ions in contaminated mine water: a review. Miner Eng 117:74–90. https://doi.org/10.1016/j.mineng.2017.12.004
Kim R, Cho HC, Han KN (2014) Behavior of anions in association with metal ions under hydrometallurgical environments: Part I. OH-effect on various cations. Mining Metall Explor 31:34–39. https://doi.org/10.1007/BF03402346
Kim R, Cho H, Jeong J et al (2020) Effect of sulfuric acid baking and caustic digestion on enhancing the recovery of rare earth elements from a refractory ore. Minerals 10:532. https://doi.org/10.3390/min10060532
Dean JA (1985) Langess handbook of chemistry. University of Tennessee, McGraw-Hill Book Company, New York
Kul M, Topkaya Y, Karakaya İ (2008) Rare earth double sulfates from pre-concentrated bastnasite. Hydrometallurgy 93:129–135. https://doi.org/10.1016/j.hydromet.2007.11.008
Jeffery GH, Basset J, Mendham J, Denney RC (1989) Textbook of quantitative chemical analysis
Mondillo N, Balassone G, Boni M et al (2019) Rare earth elements (REE) in Al- and Fe-(Oxy)-hydroxides in bauxites of provence and languedoc (Southern France): implications for the potential recovery of REEs as by-products of bauxite mining. Minerals 9:504. https://doi.org/10.3390/min9090504
Li Y, Guo W, Wu Z et al (2016) Determination of ultra-trace rare earth elements in high-salt groundwater using aerosol dilution inductively coupled plasma-mass spectrometry (ICP-MS) after iron hydroxide co-precipitation. Microchem J 126:194–199. https://doi.org/10.1016/j.microc.2015.12.006
Qi L, Zhou M-F, Malpas J, Sun M (2005) Determination of rare earth elements and Y in ultramafic rocks by ICP-MS after preconcentration using Fe(OH)3 and Mg(OH)2 coprecipitation. Geostand Geoanal Res 29:131–141. https://doi.org/10.1111/j.1751-908X.2005.tb00660.x
Acknowledgements
The financial support for this study was provided through Queen’s University Research Initiation Grant. The study benefited from the use of electron microscopy at the RMTL facility of Queen’s University, constructed with funding from CFI/ORF. Dr. Rina Kim is also grateful for the support of the Basic Research Project (Grant No. GP2020-013) of the Korea Institute of Geoscience and Mineral Resources (KIGAM), funded by the Ministry of Science and ICT of Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Additional information
The contributing editor for this article was Yongxiang Yang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sadri, F., Kim, R. & Ghahreman, A. Behavior of Light and Heavy Rare-Earth Elements in a Two-Step Fe and Al Removal Process from Rare-Earth Pregnant Leach Solutions. J. Sustain. Metall. 7, 1327–1342 (2021). https://doi.org/10.1007/s40831-021-00423-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00423-6