Abstract
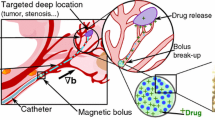
Radioembolization, a targeted treatment for advanced-stage liver cancer, is a medical procedure in which millions of radioactive microspheres (20–40 µm in diameter) are released into the blood vessels feeding liver tumors. These microspheres not only reduce blood flow to the tumors; their associated radiation kills the cancerous tissues into which they embed. While effective, this procedure also poses a threat to adjacent (non-target) healthy liver tissues. Recent research (both theoretical and experimental) has established a strong relationship between microsphere injection location (in the artery cross section) and the destination vessel, demonstrating the potential for specific vessel and tumor targeting. One such method for positioning the catheter tip, the subject of this research, is to use magnetic levitation. This paper (the second of a two-part series) details the design, fabrication, and preliminary testing of a large air gap magnetic levitator. This device, with a model-based sliding mode controller, is used to precisely position the microcatheter tip in a dynamically perfused arterial model cross section, allowing for microsphere injections from controlled locations within the cross section. Microsphere distributions were found to be significantly dependent on release location, and the large gap levitator is shown to be a viable option for catheter positioning.
Similar content being viewed by others
1 Introduction
Primary liver cancer (hepatocellular carcinoma, HCC) ranks sixth in cancer occurrence and third in cancer-related deaths worldwide; in the US, its occurrence rate is increasing faster than all other solid tumor cancers. Early stage treatments focus on ablation, resection, and complete liver transplant, while intermediate and advanced stage treatments focus on chemoembolization and medication, respectively (Mokdad et al. 2015; Forner et al. 2012).
A more recent treatment for intermediate stage HHC, radioembolization (RE), uses injected radioactive microspheres to kill cancer cells on the tumor periphery (See Fig. 1). Microspheres are typically 20–40 µm in diameter and encapsulate 90Y, a radioactive element that balances low tissue penetration (d90 = 2 mm) with high radiation dose rate and energy deposition (0.97 MeV). Treatment is performed by releasing millions of microspheres into the hepatic artery via a microcatheter positioned as distally as possible with respect to the tumor (to minimize non-target deposition to healthy liver tissue). Microspheres embed preferentially in the tumor, irradiating and killing nearby tissue (Kennedy et al. 2004; Salem and Thurston 2006a).
Two deposition scenarios of radioembolization, (A) incomplete, and (B) full tumor coverage (Richards et al. 2012)
Despite its documented benefits, RE introduces additional risks. Microspheres which embed in healthy liver tissue can cause radiation-induced liver disease, and extrahepatic deposition can result in gastrointestinal ulceration, radiation pneumonitis, and other radiation related complications (Salem and Thurston 2006b). Procedure-specific microcatheters have been developed to prevent reflux, a phenomenon that occurs when injected microspheres travel proximally (upstream) of the injection location (Hoven et al. 2015). These catheters focus on preventing retrograde flow, but still rely on being located as distally as possible to target cancerous tissue, and still allow for non-target microsphere deposition in healthy liver tissue (Xu et al. 2016). However, the distal positioning of a microcatheter is limited by its diameter, stiffness and maneuverability.
Clearly, the ability to target only cancerous tissue would provide a significant advantage in reducing undesired side effects. Previous work by Richards et al. (2012) showed a strong correlation between the microsphere injection location (in the artery cross section) and the destination vessel, demonstrating the potential for vessel specific targeting. In (Richards et al. 2012), a generalized hepatic artery model was simulated using a commercial computational fluid dynamics (CFD) package (ANSYS CFX v12.1). Simulated microspheres were released at various points in the artery cross section, and their destination vessel was recorded. The model hepatic artery system used in this study comprised a common hepatic artery (CHA), gastroduodenal artery (GDA), proper hepatic artery (PHA), left hepatic artery (LHA), right hepatic artery (RHA), and branching outlets labeled B1 through B5 (Fig. 2 bottom). The CFD prediction in Fig. 2 (top) shows a cross section of the particle release plane (x–y plane) located at the CHA inlet. The colored regions of the release plane correspond with specific downstream outlet vessels (through which the microspheres exit the model). For example: microspheres released in the yellow center of the CHA cross section will exit the model via outlet B5 (the GDA), while microspheres released to the far right pink region will exit the model via B3. These distinct regions reveal a strong repeatable correlation between microsphere release location and destination vessel.
These results were experimentally verified by constructing a physical model, scaled approximately 4.4 times to facilitate fabrication and experimental measurements. Surrogate (non-radioactive) microspheres, also approximately 4.4 times larger than those used clinically, were released from a 72.5 cm long section of 15-gauge hypodermic tubing. The tubing was supported at two axial locations using three stainless steel wires attached radially. Each wire traveled through the inlet pipe and was connected to a screw-driven positioning system, shown in Fig. 3. Release location was controlled by manually adjusting the positioning mounts, as well as by rotating the inlet tube relative to the model.
Hypodermic tubing position system used in (Richards et al. 2012), consisting of six screw-driven mounts. The hypodermic tubing was attached to the mounts using stainless steel wire
This experimental validation supports the premise that patient-specific vascular CFD models could be used to generate patient-specific microsphere release maps similar to the one shown in Fig. 2. With a CFD model based on individualized vascular anatomy and flow conditions, and given sufficient ability to control the microsphere release location, specific hepatic vessels can be targeted. These results are promising, but first a method to position clinical catheters in vivo, without penetrating or damaging the artery walls and without disrupting the laminar arterial flow, must be developed. One option for in vivo positioning, the focus of this paper, involves actuating the microcatheter tip using feedback-controlled electromagnets. Such devices are commonly referred to as active magnetic bearings (AMBs).
AMBs use pairs of electromagnets to levitate high-speed ferromagnetic rotors where bearing wear and maintenance are problematic (Fig. 4). The attractive forces exerted by each electromagnet are nonlinearly dependent on the applied coil current and separation gap: force increases with the square of coil current and is inversely proportional to the square of air gap. For this reason, AMBs are inherently unstable electromechanical systems, and require sophisticated feedback controllers for stable operation. The controller measures rotor position, compares it to the setpoint, and calculates a control signal which is sent to the power amplifier. This control action stabilizes the system, and enables the dynamic adjustment of bearing stiffness (Schweitzer 1990).
Schematic of a typical radial AMB (top) and the large air gap magnetic levitator (bottom). The typical AMB has a rotor to bearing diameter ratio (DR/DB) of nearly unity. For the proposed large air gap levitator, it is clear that DR/DB ≪ 1, which introduces additional technical challenges not associated with typical AMB configurations. In this figure, ir and Er (r = 1,…,4) are the current and voltage, respectively, for each electromagnet
In this paper, we detail the design and experimental demonstration of a novel AMB, specifically a “large air gap levitator”, for which the rotor diameter DR is significantly smaller than the bearing’s pole-to-pole diameter DB (Fig. 4). This characteristic poses two unique technical challenges not associated with standard AMB configurations. First, the large air gaps cause excessive leakage of magnetic flux around the rotor. A consequence is that flux can pass directly between adjacent poles, bypassing the rotor entirely and reducing control authority. These excessive leakage and mutual fluxes result in more complex nonlinear behavior than that described by typical AMB models (Lindlau and Knospe 2002; Banerjee et al. 2006; Smith and Weldon 1995). The second technical challenge associated with large air gap levitators is maintaining robustness and performance over a wide range of rotor position setpoints. AMBs usually regulate rotor position about the origin to provide frictionless support; thus, models linearized about the origin can be sufficient for controller design (Schweitzer and Maslen 2009; Trumper et al. 1997). Controlling a large air gap levitator with excessive leakage and mutual fluxes over a wide range of setpoints necessitates the use of nonlinear control strategies to position the tip of a RE microcatheter in a hepatic artery model that replicates in vivo flow characteristics. Two performance criteria are evaluated: the dynamic response of the microcatheter to changes in setpoint, and microsphere distributions when released from AMB-controlled locations within the model's cross section.
2 Methods
2.1 Hepatic artery model
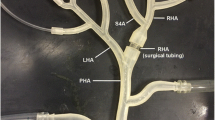
A two-generation, bifurcated hepatic artery (HA) model, designed to replicate the vessel diameters and flow rates of a portion of human HA anatomy (Hübner et al. 2000; Hirata et al. 2001; Han et al. 2002; Basciano et al. 2010; Jernigan et al. 2015), was created for experimental testing (Fig. 5). Fabricated using stereolithography from an optically transparent liquid photopolymer, this rigid planar model consists of one inlet and four terminal vessels. At the injection plane, a rectangular cross section was fabricated from optically clear acrylic sheet to aid in optical position sensing, as this reduces image distortion caused by refraction. The inlet has an ID (inner diameter) of 4 mm and is barbed to accept standard 9.5 mm OD (outer diameter) tubing. Each terminal vessel has an inner diameter of 1 mm and is connected to clear polyvinyl chloride tubing. The output of each vessel is connected to a 100 µm mesh basket filter, which allows for the collection and quantification of microspheres terminating in each vessel.
Water (approximately 20 °C) was selected for the working fluid, as its density closely matches that of human blood. As shown in Fig. 6, this working fluid is pumped through the HA model using a voltage-controlled positive displacement gear pump (Greylor Company, Cape Coral, Florida) at a flow rate of 102 mL/min, which is in the range of published measurements (Jernigan et al. 2015).
Schematic for the HA model, adapted from (Richards et al. 2012). Supply reservoir (1), collection reservoir (2), gear pumps (3), flowmeter (4), syringe pump (5), magnetic levitator (6), microcatheter (7), HA model (8), microsphere filters (9). Arrows indicate the direction of flow
2.2 Large air gap magnetic levitator design
The novel magnetic levitator used for microcatheter tip position control uses four electromagnets (two differentially driven pairs) to exert two-dimensional reluctance forces on a ferromagnetic ring attached to the microcatheter tip. The microcatheter tip position is measured optically using two linear arrays of photodetectors. A schematic of this levitator is presented in Fig. 7.
Schematic of the large air gap magnetic levitator for microcatheter tip control. a Two pairs of differentially-driven electromagnets exert controllable reluctance force on microcatheter ring. b Details of single electromagnet (1), controller (2), N-channel MOSFET (3), linear optical array (4), ferromagnetic ring (5), and laser diode (6)
A custom end-hole microcatheter (Fig. 8) was fabricated using 21.5 mm length stainless steel hypodermic tubing with a 1.07 mm OD and a 0.81 mm ID (inner diameter). To enable the application of controllable reluctance forces, a mild steel ring was attached to this length of tubing 15.6 mm from the tip. Machined on a lathe, the ring had an OD of 2 mm, an ID of 1 mm, and an axial length of 5.7 mm. For clinical use, this ring could be ensheathed in a common biocompatible polymer such as polyethylene or polytetrafluoroethylene. Current clinical microcatheters employ such materials to minimize friction and improve maneuverability. Hypodermic tubing was used for the catheter tip to provide a rigid opaque target for optical position measurements. Additionally, the rigidity of the tubing allows position measurements at the end-hole to be used for both particle release and ferromagnetic ring positons.
This tip assembly was then connected to a longer length of hypodermic access tubing by a section of flexible silicone tubing. The silicone tube was 6 mm in length and had a 1.19 mm OD and a 0.64 mm ID. To secure the access tubing to the center of the HA model without obstructing flow, a support was created from six lengths of hypodermic tubing attached to the outside circumference of the access tubing, as shown in Fig. 8. This support prevented the access tubing from shifting during experimental trials, allowing for precise control of the end-hole position.
Reluctance force generated by the electromagnets was maximized using Finite Element Method Magnetics (FEMM), a magnetic finite element analysis (FEA) software package. Electromagnet geometry was imported into FEMM, where forces in the vertical and horizontal directions were calculated for a range of currents. Typical FEA results are shown in Fig. 9.
Using an iterative design process, electromagnet geometry was optimized to provide reluctance force magnitudes ranging from 2.7 to 66.9 mN (corresponding to maximum and minimum gaps between levitator poles and microcatheter tip) for continuous coil excitations of 400 mA (the peak current rating of the 36 gauge magnetic wire used in the coils). This force range was deemed sufficient to actuate the microcatheter assembly, as the heaviest component (the steel ring) had a weight of approximately 0.65 mN; thus, the levitator was capable of lifting the microcatheter when resting on the bottom of the HA model. It should be noted that nominal coil current was less than half of the peak current to avoid thermal degradation of the magnet wire’s insulation.
Each electromagnet was made of 12 laminations of electrical steel and one electrical winding per leg. Laminations were waterjet cut from 0.020″ thick ultra-low carbon electrical steel (CMI-B, CMI Specialty Products, Bristol, CT). After waterjet cutting, the laminations were heat treated per the datasheet schedule. The windings, two per electromagnet, each consisted of approximately 700 turns of 36-gauge magnet wire, and were wound using a customized CNC (computer numerical control) milling tool. The four electromagnets were controlled individually using pulse width modulation (PWM) of four metal–oxide–semiconductor field-effect transistors (MOSFET), one per electromagnet.
Position feedback was achieved using two TSL1402R linear optical arrays (ams AG, Unterpremstaetten, Austria) pictured in Fig. 10. Each optical array consists of 256 pixels, spaced 63.5 µm on center, and functions like a digital camera; its pixels are exposed to a light source for a period of time and the analog output voltage at the end of the exposure period is proportional to light intensity. The optical sensors were illuminated by two low power laser diodes. An image of the catheter is generated when the stainless steel tip blocks the collimated laser light, thereby casting a silhouette on the optical arrays. To enhance signal-to-noise ratios, optical filters were attached to the linear optical arrays. A red color filter was used to prevent all but red light from reaching the sensor, thus blocking undesirable wavelengths from extraneous light sources. A neutral density filter was used to reduce the amount of ambient light reaching the optical arrays and to achieve a desirable laser line exposure.
The output of each optical array is a sequence of 256 analog voltages, each corresponding to the intensity of light reaching a specific pixel integrated over the exposure period. As shown in Fig. 11, a position-dependent voltage profile is generated by the silhouette of the catheter tip. To compute the microcatheter position, the sensor output is first inverted by subtracting the output voltage from 3.3 V (the maximum output voltage). Next, the inverted signal is integrated to calculate the centroid of the area beneath the curve, yielding the uncalibrated microcatheter position in pixels. The position is calibrated by moving the ferromagnetic ring against the inside of the HA model and recording the minimum and maximum uncalibrated readings from both sensors. Since the ID of the HA model is 4 mm and the OD of the ring is 2 mm, the extent of the microcatheter position is ± 1 mm in each direction.
The linear optical arrays were secured in place by a 3D printed fixture which was mounted to the levitator chassis (Figs. 12, 13). The laser modules were secured to an adjustable aluminum frame to enable alignment of the laser lines and optical arrays. The entire prototype assembly was mounted vertically to remove the effect of gravity on the catheter.
The position controller consists of three hardware components: the linear optical arrays, a STM32F407VG microcontroller (STMicroelectronics, Geneva, Switzerland), and a TPL7407L 7-channel MOSFET low side driver (Texas Instruments Incorporated, Dallas, TX). The microcontroller acquires voltage data from the optical arrays, computes the microcatheter position, calculates a control action, and updates the PWM output to the MOSFET driver. The driver then switches a 14 V regulated power supply thereby modulating the current to each electromagnet. This control loop is executed at a real-time rate of 150 Hz; simultaneously, the microcontroller transmits microcatheter position, tracking error, and control action via serial connection to a desktop computer where a custom MATLAB (The MathWorks Inc., Natick, MA) application plots the serial data in real time.
A model-based sliding mode controller was designed using the FEA model shown in Fig. 9 and implemented in software on the microcontroller. This controller was chosen for its robustness and ability to compensate for nonlinear dynamics introduced by the large air gap configuration. A detailed exposition of the design and testing of this controller can be found in (Miller et al. 2018).
2.3 Testing methodology
To quantify targeting performance, microsphere injections were made at three locations in the HA model cross section labeled A, B, and C in Fig. 14. Polyethylene microspheres (Cospheric, Santa Barbara, CA) 212–250 µm in diameter were added to 25 °C tap water to create a microsphere suspension with a density of approximately 1000 microspheres/mL. Tests were conducted by positioning the microcatheter in the desired cross-sectional location, approximately 5 mm proximal to the first bifurcation in the HA model (as indicated in Fig. 5). Eight injections of microsphere solution (0.5 mL each) were made at each cross-sectional location using a computer-controlled syringe pump with the injection rate configured such that the exit velocity of the microspheres matched the fluid velocity inside the HA model (13.5 cm/s). The microsphere solution was agitated between injections to maintain a uniform microsphere suspension.
Resulting microsphere distributions were quantified using 100 µm nylon mesh filters. Prior to each test, these filters were cleaned, dried, and inspected. Following each injection, a light source and a frosted glass element were placed under each filter, and photographs were taken from above the filter. As shown in Fig. 15, this method provided excellent contrast between the white filter background and the dark green microspheres. Microspheres were automatically counted using the image processing and analysis software ImageJ (U. S. National Institutes of Health, Bethesda, MD). The vessel outcome frequencies were then recorded for each release location.
2.4 Statistical methods
Particle frequency distribution statistics were evaluated using a χ2 test of independence. This test uses an independence model that assumes the vessel outcome and release location are independent events. Expected vessel frequencies are determined by multiplying the probability that a particle exited a particular vessel with the probability that a particle was released from a specified location. This is computed by using Eq. 1, where \({N}_{\text{l}}\) is the total number of particles released at Location \(l\), \({N}_{\text{v}}\) is the total number of particles that exited Vessel \({\text{v}}\) over all release locations, and \({N}_{{\text{tota}}{\text{l}}}\) is the total number of particles released over all locations. For example, \({f}_{\text{e,A3}}\) is the expected frequency with which particles will be observed exiting Vessel 3 given that they are released from Location A according to the independence model.
The χ2 value is computed by summing the squared standardized residuals as shown in Eq. 2. Here, \({f}_{\text{o,lv}}\) is the observed frequency for vessel \(v\) when particles are released from location \(l\). If the independence model is true, then the probability that χ2 is less than \({\chi }_{0.01}^{2}\) = 16.8 is 0.99. Thus, if χ2 is found to be greater than 16.8, then the events are likely to be dependent at a significance level of \(\alpha \) = 0.01.
The strength of the association between release location and vessel outcome was assessed using Cramér’s V, which is calculated using Eq. 3. In this equation, \(m\) is equal to the number of locations or vessels, whichever is smaller. Cramér's V is commonly used for measuring the degree of association because it is unaffected by sample size. Complete association is indicated by \(V\) = 1, while no association is indicated by \(V\) = 0.
3 Results
Initial trials were conducted to demonstrate the levitator’s positioning performance using the sliding mode controller. Figure 16 shows the closed-loop response to various steps in the x and y reference positions. Here, catheter position was regulated with an average error of 0.028 mm in both the x and y directions with an average rise time of 0.63 s. Figure 17 shows the closed-loop tracking response for a 1.2 mm diameter circular trajectory, with an average tracking error magnitude of 0.23 mm. In Figs. 16 and 17, the reference signals are indicated by dashed lines. The high frequency variations in these signals are the result of fluttering caused by the fluid flow in the HA model and the low stiffness of the silicone tubing.
The outlet vessel frequency distributions for the microsphere injections are shown in Table 1 along with marginal frequencies and column percentages. Additionally, the column percentages are plotted in the bar graph in Fig. 18. The χ2 statistic for the data in Table 1 was calculated to be 1037.3 with a Cramér's V of 0.32.
4 Discussion
As demonstrated in Fig. 16, the large air gap magnetic levitator proved capable of precisely regulating the microcatheter position with a sufficiently short rise time and low steady state error. In addition, catheter tip motion in the x and y directions are independent. In other words, a step in the x direction does not affect regulation in the y direction and vice versa. Figure 17 also demonstrates command following over a large region within the HA model cross section. Here, the speed of the controller limits the accuracy with which the catheter can track a moving trajectory. The bottom plot in Fig. 17 reveals a non-uniform pattern in the tracking error which is not observed in Fig. 16. This is likely due to the relatively slow compensation for uncertainties within the system, such as anisotropy in the stiffness of the catheter and non-laminar flow. While not required for these microsphere targeting experiments, closer tracking performance can be achieved by adjusting controller parameters, such as the switching control amplitude, to meet performance requirements. Overall, this tracking performance in the face of large air gap nonlinearities and model uncertainties is a key feature of sliding mode control and justifies the use of such a controller for this system.
The experimental results shown in Table 1 and the associated χ2 value indicate that the vessel distributions and microcatheter positions are likely to be dependent in some way (\(\alpha =0.01)\). Furthermore, the Cramér's V value calculated indicates that there is a moderately strong association between the distributions and positions; this association is obvious in Fig. 18. When the catheter is positioned 0.6 mm to the right of center (Position A), microspheres preferentially target vessels 3 and 4, with 47.4% of microspheres exiting vessel 4. Likewise, when the catheter is positioned 0.6 mm to the left (Position C), microspheres favor vessels 1 and 2, with 41.6% exiting vessel 1. When the catheter is located at the center of the HA model (Position B), the vessel outcomes more closely resemble a uniform distribution. These results corroborate the findings of (Richards et al. 2012), indicating that vessel targeting is achievable on a 1:1 scale model.
In addition to potential sources of error encountered by previous work, these results could potentially be affected by the microcatheter not being parallel with the flow, flow disturbances caused by the microcatheter resting along the artery model wall, and movement of the microcatheter during injections. The addition of the rectangular cross section to allow for optical position measurements (Fig. 5) also affects flow within the HA model. Resulting turbulence had the effect of dispersing the microspheres in the flow over longer distances (> 20 mm). The microcatheter end-hole was positioned 5 mm proximally to the first artery model bifurcation (Fig. 5), reducing the impact of such flow disturbances.
This demonstration shows that non-contact positioning of a microcatheter is indeed possible and that targeting could be achieved with patient-specific vascular modeling. If a large enough levitator could be constructed, the patient could be positioned inside the device while the procedure is being performed. The largest challenges facing this type of procedure are scale and position feedback. Reluctance force decreases with the square of distance, and therefore very large currents and magnetic fields would be required to exert appreciable force on the microcatheter. Additionally, the levitator in this paper benefits from a simple high resolution position feedback mechanism. Clinical trials would need a similarly high resolution transducer, despite the additional challenges imposed by intravascular procedures. If these challenges can be overcome, using an active magnetic bearing for targeted RE treatments may prove to be an effective procedure.
5 Conclusion
Results from microsphere injections corroborated the results of (Richards et al. 2012), demonstrating that vessel targeting is possible using a microcatheter in a 1:1 scale HA model. Despite being only 1.2 mm apart, the A and C injection locations had significantly different terminal vessel distributions. Flow disturbances were found to have a dispersing effect on the injected microspheres, thus the injection location should be located as close as possible to the target vessel.
Using the model-based sliding mode controller designed in (Miller et al. 2018) enabled precise positioning of the microcatheter tip with minimal steady-state error. The closed-loop system also exhibited acceptable command following over a large region of the HA model cross section. This, along with the microsphere injection results, demonstrates the ability for this system to meet the performance required for targeted RE therapy.
References
Banerjee, S., Prasad, D., Pal, J.: Large gap control in electromagnetic levitation. ISA Trans. 45, 215–224 (2006). https://doi.org/10.1016/S0019-0578(07)60191-8
Basciano, C.A., Kleinstreuer, C., Kennedy, A.S., Dezarn, W.A., Childress, E.: Computer modeling of controlled microsphere release and targeting in a representative hepatic artery system. Ann. Biomed. Eng. 38, 1862–1879 (2010). https://doi.org/10.1007/s10439-010-9955-z
Forner, A., Llovet, J.M., Bruix, J.: Hepatocellular carcinoma. Lancet 379, 1245–1255 (2012). https://doi.org/10.1016/S0140-6736(11)61347-0
Han, S.-H.B., Rice, S., Cohen, S.M., Reynolds, T.B., Fong, T.-L.: Duplex doppler ultrasound of the hepatic artery in patients with acute alcoholic hepatitis. J. Clin. Gastroenterol. 34, 573–577 (2002)
Hirata, M., Akbar, S.M.F., Horiike, N., Onji, M.: Noninvasive diagnosis of the degree of hepatic fibrosis using ultrasonography in patients with chronic liver disease due to hepatitis C virus. Eur. J. Clin. Investig. 31, 528–535 (2001). https://doi.org/10.1046/j.1365-2362.2001.00840.x
Hübner, G.H., Steudel, N., Kleber, G., Behrmann, C., Lotterer, E., Fleig, W.E.: Hepatic arterial blood flow velocities: assessment by transcutaneous and intravascular Doppler sonography. J. Hepatol. 32, 893–899 (2000). https://doi.org/10.1016/S0168-8278(00)80093-8
Jernigan, S.R., Osborne, J.A., Mirek, C.J., Buckner, G.: Selective internal radiation therapy: quantifying distal penetration and distribution of resin and glass microspheres in a surrogate arterial model. J. Vasc. Interv. Radiol. 26, 897–904.e2 (2015). https://doi.org/10.1016/j.jvir.2015.02.022
Kennedy, A.S., Nutting, C., Coldwell, D., Gaiser, J., Drachenberg, C.: Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int. J. Radiat. Oncol. Biol. Phys. 60, 1552–1563 (2004)
Lindlau, J.D., Knospe, C.R.: Feedback linearization of an active magnetic bearing with voltage control. IEEE Trans. Control Syst. Technol. 10, 21–31 (2002). https://doi.org/10.1109/87.974335
Miller, S.R., Zaccardo, V.M., Buckner, G.D.: A large air gap magnetic levitator for precise positioning of a clinical microcatheter: nonlinear modelling and control. Int. J. Intell. Robot. Appl. 2, 462–469 (2018). https://doi.org/10.1007/s41315-018-0077-3
Mokdad, A.A., Singal, A.G., Yopp, A.C.: Liver cancer. JAMA 314, 2701–2701 (2015). https://doi.org/10.1001/jama.2015.15425
Richards, A.L., Kleinstreuer, C., Kennedy, A.S., Childress, E., Buckner, G.D.: Experimental microsphere targeting in a representative hepatic artery system. IEEE Trans. Biomed. Eng. 59, 198–204 (2012). https://doi.org/10.1109/TBME.2011.2170195
Salem, R., Thurston, K.G.: Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 1: technical and methodologic considerations. J. Vasc. Interv. Radiol. 17, 1251–1278 (2006a). https://doi.org/10.1097/01.RVI.0000233785.75257.9A
Salem, R., Thurston, K.G.: Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies Part 2: special topics. J. Vasc. Interv. Radiol. 17, 1425–1439 (2006b). https://doi.org/10.1097/01.RVI.0000235779.88652.53
Schweitzer, G.: Magnetic bearings-applications, concepts and theory. JSME Int. J. 33, 13–18 (1990)
Schweitzer, G., Maslen, E.H.: Magnetic Bearings: Theory, Design, and Application to Rotating Machinery. Springer Science & Business Media, Berlin (2009)
Smith, R.D., Weldon, W.F.: Nonlinear control of a rigid rotor magnetic bearing system: modeling and simulation with full state feedback. IEEE Trans. Magn. 31, 973–980 (1995). https://doi.org/10.1109/20.364771
Trumper, D.L., Olson, S.M., Subrahmanyan, P.K.: Linearizing control of magnetic suspension systems. IEEE Trans. Control Syst. Technol. 5, 427–438 (1997). https://doi.org/10.1109/87.595924
van den Hoven, A.F., Lam, M.G.E.H., Jernigan, S., van den Bosch, M.A.A.J., Buckner, G.D.: Innovation in catheter design for intra-arterial liver cancer treatments results in favorable particle-fluid dynamics. J. Exp. Clin. Cancer Res. 34, 74 (2015). https://doi.org/10.1186/s13046-015-0188-8
Xu, Z., Jernigan, S., Kleinstreuer, C., Buckner, G.D.: Solid tumor embolotherapy in hepatic arteries with an anti-reflux catheter system. Ann. Biomed. Eng. 44, 1036–1046 (2016). https://doi.org/10.1007/s10439-015-1411-7
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaccardo, V.M., Miller, S.R. & Buckner, G.D. A large air gap magnetic levitator for intra-arterial positioning of a clinical microcatheter: design, fabrication, and preliminary experimental evaluation. Int J Intell Robot Appl 4, 122–131 (2020). https://doi.org/10.1007/s41315-020-00124-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41315-020-00124-5