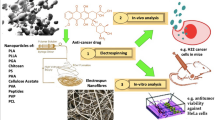
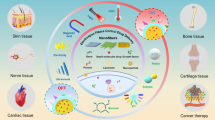
Abstract
Despite great efforts and advancement in the treatment of cancer, tumor recurrence and metastasis remain significant challenges and demand novel therapy strategies. Recently, advances in biomaterials and drug delivery systems have facilitated the development of the local therapy of cancer, among which electrospun nanofibrous scaffolds show great promise owing to their porous structure, relatively large surface area, high drug loading capacity, similarity with the native extracellular matrix, and possibility of the combination of various therapies. Here, we review this rapidly developing field of electrospun nanofibrous scaffolds as a drug delivery system for cancer local therapy, in particular addressing stimuli-responsive drug release, as well as its combination with stem cell and immune therapy. Challenges and future perspectives are also discussed.
Graphic Abstract


Copyright © 2017 Scientific Reports

(Reproduced with permission [50]. Copyright © 2015 Colloids and Surfaces B: Biointerfaces)

(Reproduced with permission [55]. Copyright © 2018 Advanced Healthcare Materials)

(Reproduced with permission [65]. Copyright © 2017 ChemMedChem)

(Reproduced with permission [69]. Copyright © 2018 MRS Advances)

(Reproduced with permission [73]. Copyright © 2015 Advanced Healthcare Materials)

(Reproduced with permission [19]. Copyright © 2018 Advanced Functional Materials)

(Reproduced with permission [98]. Copyright © 2017 ACS Nano)

(Reproduced with permission [104]. Copyright © 2019 Small)

(Reproduced with permission [115]. Copyright © 2018 Materials Horizons)
Similar content being viewed by others
References
Collaboration, GB of DC. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749.
Demicheli R, Retsky MW, Hrushesky WJM, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19:1821.
Tavare AN, Perry NJS, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130:1237.
Poláková L, Širc J, Hobzová R, Cocârţă A-I, Heřmánková E. Electrospun nanofibers for local anticancer therapy: review of in vivo activity. Int J Pharm. 2019;558:268.
Merkow R, Bilimoria K, Tomlinson J, Paruch J, Fleming J, Talamonti M, Ko C, Bentrem D. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372.
Breugom AJ, Swets M, Bosset J-F, Collette L, Sainato A, Cionini L, Glynne-Jones R, Counsell N, Bastiaannet E, van den Broek CBM, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16:200.
Qin S-Y, Zhang A-Q, Cheng S-X, Rong L, Zhang X-Z. Drug self-delivery systems for cancer therapy. Biomaterials. 2017;112:234.
Singh M, Kundu S, Reddy A, Sreekanth V, Motiani RK, Sengupta S, Srivastava A, Bajaj A. Injectable small molecule hydrogel as a potential nanocarrier for localized and sustained in vivo delivery of doxorubicin. Nanoscale. 2014;6:12849.
Cho K, Wang X, Nie S, Chen Z, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310.
Sasikala ARK, Unnithan AR, Park CH, Kim CS. Chapter 2-nanofiber-based anticancer drug delivery platform. In: Biomimetic nanoengineered materials for advanced drug delivery. 2019, pp 11–36.
Cui W, Zhou Y, Chang J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mater. 2010;11:014108.
Peng S, Li L, Hu Y, Srinivasan M, Cheng F, Chen J, Ramakrishna S. Fabrication of spinel one-dimensional architectures by single-spinneret electrospinning for energy storage applications. ACS Nano. 2015;9:1945.
Mercante LA, Scagion VP, Migliorini FL, Mattoso LHC, Correa DS. Electrospinning-based (bio)sensors for food and agricultural applications: a review. TrAC Trends Anal Chem. 2017;91:91.
Sun B, Long Y-Z, Chen Z-J, Liu S-L, Zhang H-D, Zhang J-C, Han W-P. Recent advances in flexible and stretchable electronic devices via electrospinning. J Mater Chem C. 2014;2:1209.
Taylor GI. Disintegration of water drops in an electric field. Proc R Soc Lond Ser Math Phys Sci. 1964;280:383.
Reneker DH, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7:216.
Naahidi S, Jafari M, Logan M, Wang Y, Yuan Y, Bae H, Dixon B, Chen P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol Adv. 2017;35:530.
Gupta P, Adhikary M, Kumar M, Bhardwaj N, Mandal BB. Biomimetic, osteoconductive non-mulberry silk fiber reinforced tricomposite scaffolds for bone tissue engineering. ACS Appl Mater Interfaces. 2016;8:30797.
Sasikala ARK, Unnithan AR, Thomas RG, Ko SW, Jeong YY, Park CH, Kim CS. Multifaceted implantable anticancer device for potential postsurgical breast cancer treatment: a single platform for synergistic inhibition of local regional breast cancer recurrence, surveillance, and healthy breast reconstruction. Adv Funct Mater. 2018;28:1704793.
GhavamiNejad A, Sasikala ARK, Unnithan AR, Thomas RG, Jeong YY, Vatankhah-Varnoosfaderani M, Stadler FJ, Park CH, Kim CS. Mussel-inspired electrospun smart magnetic nanofibers for hyperthermic chemotherapy. Adv Funct Mater. 2015;25:2867.
Lim CT. Nanofiber technology: current status and emerging developments. Prog Polym Sci. 2017;70:1.
Haider A, Haider S, Kang I-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab J Chem. 2018;11:1165.
Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989.
Poncelet D, de Vos P, Suter N, Jayasinghe SN. Bio-electrospraying and cell electrospinning: progress and opportunities for basic biology and clinical sciences. Adv Healthc Mater. 2012;1:27.
Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release. 2014;185:12.
Brudno Y, Mooney DJ. On-demand drug delivery from local depots. J Control Release. 2015;219:8.
Wolinsky JB, Colson YL, Grinstaff MW. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Control Release. 2012;159:14.
Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591.
Mbeunkui F, Johann DJ. Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63:571.
Thews O, Riemann A. Tumor pH and metastasis: a malignant process beyond hypoxia. Cancer Metastasis Rev. 2019;38:113.
Lindeman LR, Randtke EA, High RA, Jones KM, Howison CM, Pagel MD. A comparison of exogenous and endogenous CEST MRI methods for evaluating in vivo pH. Magn Reson Med. 2018;79:2766.
Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449.
Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, Wang J, Wen D, Zhang Y, Lu Y. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol. 2019;14:89.
Tsuchiya Y, Sawada S, Yoshioka I, Ohashi Y, Matsuo M, Harimaya Y, Tsukada K, Saiki I. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133:547.
Camara O, Kavallaris A, Nöschel H, Rengsberger M, Jörke C, Pachmann K. Seeding of epithelial cells into circulation during surgery for breast cancer: the fate of malignant and benign mobilized cells. World J Surg Oncol. 2006;4:67.
Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev. 2017;36:159.
Zeeshan MA, Shou K, Sivaraman KM, Wuhrmann T, Pané S, Pellicer E, Nelson BJ. Nanorobotic drug delivery: if I only had a heart. Mater Today. 2011;14:54.
Padma VV. An overview of targeted cancer therapy. BioMedicine. 2015;5:19.
Moses MA, Brem H, Langer R. Advancing the field of drug delivery: taking aim at cancer. Cancer Cell. 2003;4:337.
Khodadadi M, Alijani S, Montazeri M, Esmaeilizadeh N, Sadeghi-Soureh S, Pilehvar-Soltanahmadi Y. Recent advances in electrospun nanofiber-mediated drug delivery strategies for localized cancer chemotherapy. J Biomed Mater Res A. 2020;108:1444.
Rasouli S, Montazeri M, Mashayekhi S, Sadeghi-Soureh S, Dadashpour M, Mousazadeh H, Nobakht A, Zarghami N, Pilehvar-Soltanahmadi Y. Synergistic anticancer effects of electrospun nanofiber-mediated codelivery of Curcumin and Chrysin: possible application in prevention of breast cancer local recurrence. J Drug Deliv Sci Technol. 2020;55:101402.
Monterrubio C, Pascual-Pasto G, Cano F, Vila-Ubach M, Manzanares A, Schaiquevich P, Tornero JA, Sosnik A, Mora J, Carcaboso AM. SN-38-loaded nanofiber matrices for local control of pediatric solid tumors after subtotal resection surgery. Biomaterials. 2016;79:69.
Kaplan JA, Liu R, Freedman JD, Padera R, Schwartz J, Colson YL, Grinstaff MW. Prevention of lung cancer recurrence using cisplatin-loaded superhydrophobic nanofiber meshes. Biomaterials. 2016;76:273.
Ramachandran R, Junnuthula VR, Gowd GS, Ashokan A, Thomas J, Peethambaran R, Thomas A, Unni AKK, Panikar D, Nair SV, et al. Theranostic 3-dimensional nano brain-implant for prolonged and localized treatment of recurrent glioma. Sci Rep. 2017;7:43271.
Borteh HM, Gallovic MD, Sharma S, Peine KJ, Miao S, Brackman DJ, Gregg K, Xu Y, Guo X, Guan J, et al. Electrospun acetalated dextran scaffolds for temporal release of therapeutics. Langmuir. 2013;29:7957.
Graham-Gurysh E, Moore KM, Satterlee AB, Sheets KT, Lin F-C, Bachelder EM, Miller CR, Hingtgen SD, Ainslie KM. Sustained delivery of doxorubicin via acetalated dextran scaffold prevents glioblastoma recurrence after surgical resection. Mol Pharm. 2018;15:1309.
Ranganath SH, Wang C-H. Biodegradable microfiber implants delivering paclitaxel for post-surgical chemotherapy against malignant glioma. Biomaterials. 2008;29:2996.
Qiu K, He C, Feng W, Wang W, Zhou X, Yin Z, Chen L, Wang H, Mo X. Doxorubicin-loaded electrospun poly(l-lactic acid)/mesoporous silica nanoparticles composite nanofibers for potential postsurgical cancer treatment. J Mater Chem B. 2013;1:4601.
Yuan Z, Pan Y, Cheng R, Sheng L, Wu W, Pan G, Feng Q, Cui W. Doxorubicin-loaded mesoporous silica nanoparticle composite nanofibers for long-term adjustments of tumor apoptosis. Nanotechnology. 2016;27:245101.
Zhao X, Zhao J, Lin ZYW, Pan G, Zhu Y, Cheng Y, Cui W. Self-coated interfacial layer at organic/inorganic phase for temporally controlling dual-drug delivery from electrospun fibers. Colloids Surf B Biointerfaces. 2015;130:1.
Jain S, Meka SRK, Chatterjee K. Engineering a piperine eluting nanofibrous patch for cancer treatment. ACS Biomater Sci Eng. 2016;2:1376.
Xu X, Chen X, Xu X, Lu T, Wang X, Yang L, Jing X. BCNU-loaded PEG–PLLA ultrafine fibers and their in vitro antitumor activity against Glioma C6 cells. J Controlled Release. 2006;114:307.
Yang G, Wang J, Wang Y, Li L, Guo X, Zhou S. An implantable active-targeting micelle-in-nanofiber device for efficient and safe cancer therapy. ACS Nano. 2015;9:1161.
Wong AD, Ye M, Ulmschneider MB, Searson PC. Quantitative analysis of the enhanced permeation and retention (epr) effect. PLoS One. 2015;10:e0123461.
Xia G, Zhang H, Cheng R, Wang H, Song Z, Deng L, Huang X, Santos HA, Cui W. Localized controlled delivery of gemcitabine via microsol electrospun fibers to prevent pancreatic cancer recurrence. Adv Healthc Mater. 2018;7:1800593.
Wen Y, Wen P, Hu T-G, Linhardt RJ, Zong M-H, Wu H, Chen Z-Y. Encapsulation of phycocyanin by prebiotics and polysaccharides-based electrospun fibers and improved colon cancer prevention effects. Int J Biol Macromol. 2020;149:672.
Yang K-N, Zhang C-Q, Wang W, Wang PC, Zhou J-P, Liang X-J. pH-responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment. Cancer Biol Med. 2014;11:34.
Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991.
Zhao X, Yuan Z, Yildirimer L, Zhao J, Lin ZY, Cao Z, Pan G, Cui W. Tumor-triggered controlled drug release from electrospun fibers using inorganic caps for inhibiting cancer relapse. Small. 2015;11:4284.
Yuan Z, Zhao X, Zhao J, Pan G, Qiu W, Wang X, Zhu Y, Zheng Q, Cui W. Synergistic mediation of tumor signaling pathways in hepatocellular carcinoma therapy via dual-drug-loaded pH-responsive electrospun fibrous scaffolds. J Mater Chem B. 2015;3:3436.
Zhao J, Jiang S, Zheng R, Zhao X, Chen X, Fan C, Cui W. Smart electrospun fibrous scaffolds inhibit tumor cells and promote normal cell proliferation. RSC Adv. 2014;4:51696.
Yuan Z, Zhao J, Yang Z, Wang X, Zheng Q, Cui W. Integrated therapy on residual tumor after palliative operation using dual-phase drug releasing electrospun fibrous scaffolds. J Control Release Off J Control Release Soc. 2015;213:e151.
Zhao J, Liu S, Li B, Yang H, Fan C, Cui W. Stable acid-responsive electrospun biodegradable fibers as drug carriers and cell scaffolds. Macromol Biosci. 2013;13:885.
Yuan Z, Wu W, Zhang Z, Sun Z, Cheng R, Pan G, Wang X, Cui W. In situ adjuvant therapy using a responsive doxorubicin-loaded fibrous scaffold after tumor resection. Colloids Surf B Biointerfaces. 2017;158:363.
Wang W, Cheng Y, Li Y, Zhou H, Xu L-P, Wen Y, Zhao L, Zhang X. Enrichment and viability inhibition of circulating tumor cells on a dual acid-responsive composite nanofiber film. ChemMedChem. 2017;12:529.
Fu Y, Li X, Ren Z, Mao C, Han G. Multifunctional electrospun nanofibers for enhancing localized cancer treatment. Small. 2018;14:1801183.
Jiang J, Xie J, Ma B, Bartlett DE, Xu A, Wang C-H. Mussel-inspired protein-mediated surface functionalization of electrospun nanofibers for pH-responsive drug delivery. Acta Biomater. 2014;10:1324.
Zhang Z, Wu Y, Kuang G, Liu S, Zhou D, Chen X, Jing X, Huang Y. Pt(IV) prodrug-backboned micelle and DCA loaded nanofibers for enhanced local cancer treatment. J Mater Chem B. 2017;5:2115.
Li D, Chen Y, Zhang Z, Chen M. Mesoporous nanofibers mediated targeted anti-cancer drug delivery. MRS Adv. 2018;3:2991.
Demirci S, Celebioglu A, Aytac Z, Uyar T. pH-responsive nanofibers with controlled drug release properties. Polym Chem. 2014;5:2050.
Yan E, Jiang J, Yang X, Fan L, Wang Y, An Q, Zhang Z, Lu B, Wang D, Zhang D. pH-sensitive core-shell electrospun nanofibers based on polyvinyl alcohol/polycaprolactone as a potential drug delivery system for the chemotherapy against cervical cancer. J Drug Deliv Sci Technol. 2020;55:101455.
Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655.
Li L, Yang G, Zhou G, Wang Y, Zheng X, Zhou S. Thermally switched release from a nanogel-in-microfiber device. Adv Healthc Mater. 2015;4:1658.
Slemming-Adamsen P, Song J, Dong M, Besenbacher F, Chen M. In situ cross-linked pnipam/gelatin nanofibers for thermo-responsive drug release. Macromol Mater Eng. 2015;300:1226.
Zhang H, Niu Q, Wang N, Nie J, Ma G. Thermo-sensitive drug controlled release PLA core/PNIPAM shell fibers fabricated using a combination of electrospinning and UV photo-polymerization. Eur Polym J. 2015;71:440.
Cicotte KN, Reed JA, Nguyen PAH, De Lora JA, Hedberg-Dirk EL, Canavan HE. Optimization of electrospun poly(N-isopropyl acrylamide) mats for the rapid reversible adhesion of mammalian cells. Biointerphases. 2017;12:02C417.
Hervault A, Kim Thanh NT. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale. 2014;6:11553.
Canfarotta F, Piletsky SA. Engineered magnetic nanoparticles for biomedical applications. Adv Healthc Mater. 2014;3:160.
Di Masi S, Garcia Cruz A, Canfarotta F, Cowen T, Marote P, Malitesta C, Piletsky SA. Synthesis and application of ion-imprinted nanoparticles in electrochemical sensors for copper(II) determination. ChemNanoMat. 2019;5:754.
Lin T-C, Lin F-H, Lin J-C. In vitro feasibility study of the use of a magnetic electrospun chitosan nanofiber composite for hyperthermia treatment of tumor cells. Acta Biomater. 2012;8:2704.
Lin T-C, Lin F-H, Lin J-C. In vitro characterization of magnetic electrospun IDA-grafted chitosan nanofiber composite for hyperthermic tumor cell treatment. J Biomater Sci Polym Ed. 2013;24:1152.
Huang C, Soenen SJ, Rejman J, Trekker J, Chengxun L, Lagae L, Ceelen W, Wilhelm C, Demeester J, Smedt SCD. Magnetic electrospun fibers for cancer therapy. Adv Funct Mater. 2012;22:2479.
Feng Z-Q, Shi C, Zhao B, Wang T. Magnetic electrospun short nanofibers wrapped graphene oxide as a promising biomaterials for guiding cellular behavior. Mater Sci Eng C. 2017;81:314.
Song C, Wang X-X, Zhang J, Nie G-D, Luo W-L, Fu J, Ramakrishna S, Long Y-Z. Electric field-assisted in situ precise deposition of electrospun γ-Fe2O3/polyurethane nanofibers for magnetic hyperthermia. Nanoscale Res Lett. 2018;13:273.
Radmansouri M, Bahmani E, Sarikhani E, Rahmani K, Sharifianjazi F, Irani M. Doxorubicin hydrochloride—loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int J Biol Macromol. 2018;116:378.
Niiyama E, Uto K, Lee CM, Sakura K, Ebara M. Hyperthermia nanofiber platform synergized by sustained release of paclitaxel to improve antitumor efficiency. Adv Healthc Mater. 2019;8:1900102.
Ercole F, Davis TP, Evans RA. Photo-responsive systems and biomaterials: photochromic polymers, light-triggered self-assembly, surface modification, fluorescence modulation and beyond. Polym Chem. 2010;1:37.
Appidi T, Pemmaraju DB, Khan RA, Alvi SB, Srivastava R, Pal M, Khan N, Rengan AK. Light-triggered selective ROS-dependent autophagy by bioactive nanoliposomes for efficient cancer theranostics. Nanoscale. 2020;12:2028.
Xiao W, Chen W-H, Xu X-D, Li C, Zhang J, Zhuo R-X, Zhang X-Z. Design of a cellular-uptake-shielding “plug and play” template for photo controllable drug release. Adv Mater. 2011;23:3526.
Xu X, Zeng Z, Huang Z, Sun Y, Huang Y, Chen J, Ye J, Yang H, Yang C, Zhao C. Near-infrared light-triggered degradable hyaluronic acid hydrogel for on-demand drug release and combined chemo-photodynamic therapy. Carbohydr Polym. 2020;229:115394.
Fu G-D, Xu L-Q, Yao F, Li G-L, Kang E-T. Smart nanofibers with a photoresponsive surface for controlled release. ACS Appl Mater Interfaces. 2009;1:2424.
Wei X, Liu C, Wang Z, Luo Y. 3D printed core-shell hydrogel fiber scaffolds with NIR-triggered drug release for localized therapy of breast cancer. Int J Pharm. 2020;580:119219.
Raza A, Hayat U, Rasheed T, Bilal M, Iqbal HMN. “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: a review. J Mater Res Technol. 2019;8:1497.
Cheng M, Wang H, Zhang Z, Li N, Fang X, Xu S. Gold nanorod-embedded electrospun fibrous membrane as a photothermal therapy platform. ACS Appl Mater Interfaces. 2014;6:1569.
Park JH, Seo H, Kim DI, Choi JH, Son JH, Kim J, Moon GD, Hyun DC. Gold nanocage-incorporated poly(ε-caprolactone) (pcl) fibers for chemophotothermal synergistic cancer therapy. Pharmaceutics. 2019;11:60.
Mauro N, Scialabba C, Pitarresi G, Giammona G. Enhanced adhesion and in situ photothermal ablation of cancer cells in surface-functionalized electrospun microfiber scaffold with graphene oxide. Int J Pharm. 2017;526:167.
Chen Y, Li C, Hou Z, Huang S, Liu B, He F, Luo L, Lin J. Polyaniline electrospinning composite fibers for orthotopic photothermal treatment of tumors in vivo. New J Chem. 2015;39:4987.
Wang X, Lv F, Li T, Han Y, Yi Z, Liu M, Chang J, Wu C. Electrospun micropatterned nanocomposites incorporated with Cu2s nanoflowers for skin tumor therapy and wound healing. ACS Nano. 2017;11:11337.
Kim Y-J, Ebara M, Aoyagi T. A smart hyperthermia nanofiber with switchable drug release for inducing cancer apoptosis. Adv Funct Mater. 2013;23:5753.
Sasikala ARK, Unnithan AR, Yun Y-H, Park CH, Kim CS. An implantable smart magnetic nanofiber device for endoscopic hyperthermia treatment and tumor-triggered controlled drug release. Acta Biomater. 2016;31:122.
Zhang Y, Yarin AL. Stimuli-responsive copolymers of n-isopropyl acrylamide with enhanced longevity in water for micro- and nanofluidics, drug delivery and non-woven applications. J Mater Chem. 2009;19:4732.
Li H, Sang Q, Wu J, Williams GR, Wang H, Niu S, Wu J, Zhu L-M. Dual-responsive drug delivery systems prepared by blend electrospinning. Int J Pharm. 2018;543:1.
Li H, Liu K, Williams GR, Wu J, Wu J, Wang H, Niu S, Zhu L-M. Dual temperature and pH responsive nanofiber formulations prepared by electrospinning. Colloids Surf B Biointerfaces. 2018;171:142.
Xiao J, Cheng L, Fang T, Zhang Y, Zhou J, Cheng R, Tang W, Zhong X, Lu Y, Deng L, et al. Nanoparticle-embedded electrospun fiber–covered stent to assist intraluminal photodynamic treatment of oesophageal cancer. Small. 2019;15:1904979.
Loebinger MR, Janes SM. Stem cells as vectors for antitumour therapy. Thorax. 2010;65:362.
Hu Q, Sun W, Wang J, Ruan H, Zhang X, Ye Y, Shen S, Wang C, Lu W, Cheng K, et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat Biomed Eng. 2018;2:831.
Bu L-L, Yan J, Wang Z, Ruan H, Chen Q, Gunadhi V, Bell RB, Gu Z. Advances in drug delivery for post-surgical cancer treatment. Biomaterials. 2019;219:119182.
Bagó JR, Pegna GJ, Okolie O, Mohiti-Asli M, Loboa EG, Hingtgen SD. Electrospun nanofibrous scaffolds increase the efficacy of stem cell-mediated therapy of surgically resected glioblastoma. Biomaterials. 2016;90:116.
Bagó JR, Okolie O, Dumitru R, Ewend MG, Parker JS, Werff RV, Underhill TM, Schmid RS, Miller CR, Hingtgen SD. Tumor-homing cytotoxic human induced neural stem cells for cancer therapy. Sci Transl Med. 2017;9:aah6510.
Bagó JR, Pegna GJ, Okolie O, Hingtgen SD. Fibrin matrices enhance the transplant and efficacy of cytotoxic stem cell therapy for post-surgical cancer. Biomaterials. 2016;84:42.
Masarova L, Kantarjian H, Garcia-Mannero G, Ravandi F, Sharma P, Daver N. Harnessing the immune system against leukemia: monoclonal antibodies and checkpoint strategies for AML. Immunotherapy 2017, pp. 73–95.
El-Mesery M, Trebing J, Schäfer V, Weisenberger D, Siegmund D, Wajant H. CD40-directed scFv-TRAIL fusion proteins induce CD40-restricted tumor cell death and activate dendritic cells. Cell Death Dis. 2013;4:e916.
Moradi-Kalbolandi S, Hosseinzade A, Salehi M, Merikhian P, Farahmand L. Monoclonal antibody-based therapeutics, targeting the epidermal growth factor receptor family: from herceptin to Pan HER. J Pharm Pharmacol. 2018;70:841.
Sandin LC, Tötterman TH, Mangsbo SM. Local immunotherapy based on agonistic CD40 antibodies effectively inhibits experimental bladder cancer. Oncoimmunology. 2014;3:e27400.
Liu X, Zhang H, Cheng R, Gu Y, Yin Y, Sun Z, Pan G, Deng Z, Yang H, Deng L, et al. An immunological electrospun scaffold for tumor cell killing and healthy tissue regeneration. Mater Horiz. 2018;5:1082.
Acknowledgements
This work was supported by the Natural Science Foundation of China (81930051 and 51873107) and Shanghai Talent Development Fund (2018099).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhao, J., Cui, W. Functional Electrospun Fibers for Local Therapy of Cancer. Adv. Fiber Mater. 2, 229–245 (2020). https://doi.org/10.1007/s42765-020-00053-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-020-00053-9