Abstract
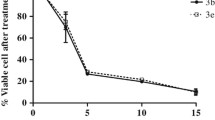
The search for new compounds with activity against Paracoccidioides, etiologic agents of Paracoccidioidomycosis (PCM), is extremely necessary due to the current scenario of the available therapeutic arsenal. Treatment is restricted to three classes of antifungals with side effects. Curcumin is a polyphenol with antifungal effects that is extracted from Curcuma longa. The present work aimed to evaluate the activity of curcumin in different species of Paracoccidioides and to evaluate the potential molecular targets of curcumin using computational strategies. In addition, interactions with classic antifungals used in the treatment of PCM were evaluated. Curcumin inhibits the growth of Paracoccidioides spp. exerting a fungicidal effect. The combination of curcumin with amphotericin B, co-trimoxazole, and itraconazole showed a synergistic or additive interaction. Molecular targets as superoxide dismutase, catalase, and isocitrate lyase were proposed based on in silico approaches. Curcumin affects the fungal plasma membrane and increases the production of reactive oxygen species. Therefore, curcumin is a good alternative for the treatment of PCM.





Similar content being viewed by others
References
Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC (2017) Neglected endemic mycoses. Lancet Infect Dis 17:e367–e377. https://doi.org/10.1016/S1473-3099(17)30306-7
Shikanai-Yasuda MA, Mendes RP, Colombo AL, Queiroz-Telles F de, Kono ASG, Paniago AMM, Nathan A, Valle ACF do, Bagagli E, Benard G, Ferreira MS, Teixeira M de M, Silva-Vergara ML, Pereira RM, Cavalcante R de S, Hahn R, Durlacher RR, Khoury Z, Camargo ZP de, Moretti ML, Martinez R (2017) Brazilian guidelines for the clinical management of paracoccidioidomycosis. Revista da Sociedade Brasileira de Medicina Tropical 50:715–740. https://doi.org/10.1590/0037-8682-0230-2017
Shikanai-Yasuda MA (2015) Paracoccidioidomycosis treatment. Rev Inst Med Trop Sao Paulo 57:31–37. https://doi.org/10.1590/S0036-46652015000700007
Travassos LR, Taborda CP, Colombo AL (2008) Treatment options for paracoccidioidomycosis and new strategies investigated. Expert Rev Anti Infect Ther 6:251–262. https://doi.org/10.1586/14787210.6.2.251
Freitas e Silva KS, C. Silva L, Gonçales RA, Neves BJ, Soares CMA, Pereira M (2020) Setting new routes for antifungal drug discovery against pathogenic fungi. CPD 26:1509–1520. https://doi.org/10.2174/1381612826666200317125956
Negri M, Salci T, Shinobu-Mesquita C, Capoci I, Svidzinski T, Kioshima E (2014) Early state research on antifungal natural products. Molecules 19:2925–2956. https://doi.org/10.3390/molecules19032925
Hewlings S, Kalman D (2017) Curcumin: a review of its’ effects on human health. Foods 6:92. 10.3390/foods6100092
Zorofchian Moghadamtousi S, Abdul Kadir H, Hassandarvish P, Tajik H, Abubakar S, Zandi K (2014) A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int 2014:1–12. https://doi.org/10.1155/2014/186864
Soleimani V, Sahebkar A, Hosseinzadeh H (2018) Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother Res 32:985–995. https://doi.org/10.1002/ptr.6054
Osawa T (2007) Nephroprotective and hepatoprotective effects of curcuminois. In: Aggarwal BB, Surh Y-J, Shishodia S (eds) The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer, Boston, MA, pp 407–423
Mohammadi A, Blesso CN, Barreto GE, Banach M, Majeed M, Sahebkar A (2019) Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J Nutr Biochem 66:1–16. https://doi.org/10.1016/j.jnutbio.2018.12.005
Ziegler S, Pries V, Hedberg C, Waldmann H (2013) Target identification for small bioactive molecules: finding the needle in the haystack. Angew Chem Int Ed 52:2744–2792. https://doi.org/10.1002/anie.201208749
Chen C, Huang H, Wu CH (2017) Protein bioinformatics databases and resources. Methods Mol Biol 1558:3–39. https://doi.org/10.1007/978-1-4939-6783-4_1
Comess KM, McLoughlin SM, Oyer JA, Richardson PL, Stöckmann H, Vasudevan A, Warder SE (2018) Emerging approaches for the identification of protein targets of small molecules - a practitioners’ perspective. J Med Chem 61:8504–8535. https://doi.org/10.1021/acs.jmedchem.7b01921
The UniProt Consortium (2017) UniProt: the universal protein knowledgebase. Nucleic Acids Res 45:D158–D169. https://doi.org/10.1093/nar/gkw1099
Gilson MK, Liu T, Baitaluk M, Nicola G, Hwang L, Chong J (2016) BindingDB in 2015: a public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res 44:D1045-1053. https://doi.org/10.1093/nar/gkv1072
Gao Z, Li H, Zhang H, Liu X, Kang L, Luo X, Zhu W, Chen K, Wang X, Jiang H (2008) PDTD: a web-accessible protein database for drug target identification. BMC Bioinformatics 9:104. https://doi.org/10.1186/1471-2105-9-104
DrugBank 5.0: a major update to the DrugBank database for 2018 - PubMed. https://pubmed.ncbi.nlm.nih.gov/29126136/. Accessed 22 Jul 2020
Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, Lai L, Pei J, Li H (2017) PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res 45:W356–W360. https://doi.org/10.1093/nar/gkx374
Xu T, Ma C, Fan S, Deng N, Lian Y, Tan L, Du W, Zhang S, Liu S, Ren B, Li Z, Wang Q, Wang X, Cheng F (2018) Systematic understanding of the mechanism of baicalin against ischemic stroke through a network pharmacology approach. Evid Based Complement Alternat Med 2018. https://doi.org/10.1155/2018/2582843
Xu T, Ma C, Fan S, Deng N, Lian Y, Tan L, Du W, Zhang S, Liu S, Ren B, Li Z, Wang Q, Wang X, Cheng F (2018) Systematic understanding of the mechanism of baicalin against ischemic stroke through a network pharmacology approach. Evid Based Complement Alternat Med 2018. https://doi.org/10.1155/2018/2582843
Silva LC, Neves BJ, Gomes MN, Melo-Filho CC, Soares CM, Andrade CH, Pereira M (2018) Computer-aided identification of novel anti-paracoccidioidomycosis compounds. Future Microbiol 13:1523–1535. https://doi.org/10.2217/fmb-2018-0175
do Carmo Silva L, Miranda MACM, de Freitas JV, Ferreira SFA, de Oliveira Lima EC, de Oliveira CMA, Kato L, Terezan AP, Rodriguez AFR, Faria FSEDV, de Almeida Soares CM, Pereira M (2020) Antifungal activity of Copaíba resin oil in solution and nanoemulsion against Paracoccidioides spp. Braz J Microbiol 51:125–134. https://doi.org/10.1007/s42770-019-00201-3
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, Von Mering C (2017) The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res 45:D362–D368. https://doi.org/10.1093/nar/gkw937
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, De Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:W296–W303. https://doi.org/10.1093/nar/gky427
Rose PW, Prlić A, Altunkaya A, Bi C, Bradley AR, Christie CH, Di Costanzo L, Duarte JM, Dutta S, Feng Z, Green RK, Goodsell DS, Hudson B, Kalro T, Lowe R, Peisach E, Randle C, Rose AS, Shao C, Tao YP, Valasatava Y, Voigt M, Westbrook JD, Woo J, Yang H, Young JY, Zardecki C, Berman HM, Burley SK (2017) The RCSB protein data bank: integrative view of protein, gene and 3D structural information. Nucleic Acids Res 45:D271–D281. https://doi.org/10.1093/nar/gkw1000
Bhattacharya D, Nowotny J, Cao R, Cheng J (2016) 3Drefine: an interactive web server for efficient protein structure refinement. Nucleic Acids Res 44:W406–W409. https://doi.org/10.1093/nar/gkw336
Bhattacharya D, Cheng J (2013) 3Drefine: Consistent protein structure refinement by optimizing hydrogen bonding network and atomic-level energy minimization. Proteins: Structure. Function, and Bioinformatics 81:119–131. https://doi.org/10.1002/prot.24167
Anandakrishnan R, Aguilar B, Onufriev AV (2012) H++ 3.0: automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res 40:W537–W541. https://doi.org/10.1093/nar/gks375
Williams CJ, Headd JJ, Moriarty NW, Prisant MG, Videau LL, Deis LN, Verma V, Keedy DA, Hintze BJ, Chen VB, Jain S, Lewis SM, Arendall WB, Snoeyink J, Adams PD, Lovell SC, Richardson JS, Richardson DC (2018) MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci 27:293–315. https://doi.org/10.1002/pro.3330
Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66:12–21. https://doi.org/10.1107/S0907444909042073
Wang Y, Bryant SH, Cheng T, Wang J, Gindulyte A, Shoemaker BA, Thiessen PA, He S, Zhang J (2017) PubChem BioAssay: 2017 update. Nucleic Acids Res 45:D955–D963. https://doi.org/10.1093/nar/gkw1118
Fourches D, Muratov E, Tropsha A (2016) Trust, but verify II: a practical guide to chemogenomics data curation. J Chem Inf Model 56:1243–1252. https://doi.org/10.1021/acs.jcim.6b00129
OMEGA v.3.0.0.1: OpenEye Scientific Software, Santa Fe, NM. http://www.eyesopen.com. Accessed 10 Dec 2020
Jakalian A, Jack DB, Bayly CI (2002) Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II Parameterization and validation. J Comput Chem 23:1623–1641. https://doi.org/10.1002/jcc.10128
QUACPAC v.1.7.0.2: OpenEye Scientific Software, Santa Fe, NM. http://www.eyesopen.com. Accessed 10 Dec 2020
(2017) OEDocking v.3.2.0: OpenEye Scientific Software, Santa Fe, NM, USA. http://www.eyesopen.com. Accessed 10 Dec 2020
McGann M (2012) FRED and HYBRID docking performance on standardized datasets. J Comput Aided Mol Des 26:897–906. https://doi.org/10.1007/s10822-012-9584-8
Tamayo D, Muñoz JF, Lopez Á, Urán M, Herrera J, Borges CL, Restrepo Á, Soares CM, Taborda CP, Almeida AJ, McEwen JG, Hernández O (2016) Identification and analysis of the role of superoxide dismutases isoforms in the pathogenesis of Paracoccidioides spp. PLoS Negl Trop Dis 10:e0004481 . https://doi.org/10.1371/journal.pntd.0004481
Tamayo D, Muñoz JF, Almeida AJ, Puerta JD, Restrepo Á, Cuomo CA, McEwen JG, Hernández O (2017) Paracoccidioides spp. catalases and their role in antioxidant defense against host defense responses. Fungal Genet Biol 100:22–32. https://doi.org/10.1016/j.fgb.2017.01.005
Dunn MF, Ramirez-Trujillo JA, Hernandez-Lucas I (2009) Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology 155:3166–3175. https://doi.org/10.1099/mic.0.030858-0
Lorenz MC, Fink GR (2001) The glyoxylate cycle is required for fungal virulence. Nature 412:83–86. https://doi.org/10.1038/35083594
Luzzatto L, Battistuzzi G (1985) Glucose-6-phosphate dehydrogenase. In: Harris H, Hirschhorn K (eds) Advances in human genetics 14. Springer, Boston, MA, pp 217–329
Chagas RF, Bailão AM, Fernandes KF, Winters MS, Pereira M, de Soares CM, A (2010) Purification of Paracoccidioides brasiliensis catalase P: subsequent kinetic and stability studies. J Biochem 147:345–351. https://doi.org/10.1093/jb/mvp182
Schloss JV, Cleland WW (1982) Inhibition of isocitrate lyase by 3-nitropropionate, a reaction-intermediate analog. Biochemistry 21:4420–4427. https://doi.org/10.1021/bi00261a035
Shin ES, Park J, Shin J-M, Cho D, Cho SY, Shin DW, Ham M, Kim JB, Lee TR (2008) Catechin gallates are NADP+-competitive inhibitors of glucose-6-phosphate dehydrogenase and other enzymes that employ NADP+ as a coenzyme. Bioorg Med Chem 16:3580–3586. https://doi.org/10.1016/j.bmc.2008.02.030
e Silva KS, da S Neto BR, Zambuzzi-Carvalho PF, de Oliveira CM, Pires LB, Kato L, Bailão AM, Parente-Rocha JA, Hernández O, Ochoa JG, de A Soares CM, Pereira M (2018) Response of Paracoccidioides lutzii to the antifungal camphene thiosemicarbazide determined by proteomic analysis. Future Microbiol 13:1473–1496. https://doi.org/10.2217/fmb-2018-0176
Lee W, Lee DG (2014) An antifungal mechanism of curcumin lies in membrane-targeted action within candida albicans. IUBMB Life 66:780–785. https://doi.org/10.1002/iub.1326
da Silva LS, Barbosa UR, Silva L do C, Soares CM, Pereira M, da Silva RA (2019) Identification of a new antifungal compound against isocitrate lyase of Paracoccidioides brasiliensis. Future Microbiol 14:1589–1606. https://doi.org/10.2217/fmb-2019-0166
Bueno PSA, Rodrigues FAV, Santos JL, Canduri F, Biavatti DC, Pimentel AL, Bagatin MC, Kioshima ÉS, de Freitas Gauze G, Seixas FAV (2019) New inhibitors of homoserine dehydrogenase from Paracoccidioides brasiliensis presenting antifungal activity. J Mol Model 25:325. https://doi.org/10.1007/s00894-019-4221-2
Santos GD, Ferri PH, Santos SC, Bao SN, Soares CMA, Pereira M (2007) Oenothein B inhibits the expression of PbFKS1 transcript and induces morphological changes in Paracoccidioides brasiliensis. Med Mycol 45:609–618. https://doi.org/10.1080/13693780701502108
Prado RS do, Alves RJ, Oliveira CMA de, Kato L, Silva RA da, Quintino GO, do Desterro Cunha S, de Almeida Soares CM, Pereira M (2014) Inhibition of Paracoccidioides lutzii Pb01 isocitrate lyase by the natural compound argentilactone and its semi-synthetic derivatives. PLoS ONE 9:e94832. https://doi.org/10.1371/journal.pone.0094832
Curcumin enhances the activity of fluconazole against Cryptococcus gattii-induced cryptococcosis infection in mice - Silva - 2016 - Journal of Applied Microbiology - Wiley Online Library. https://sfamjournals.onlinelibrary.wiley.com/doi/full/https://doi.org/10.1111/jam.12966. Accessed 13 Jul 2020
Martins CVB, da Silva DL, Neres ATM, Magalhaes TFF, Watanabe GA, Modolo LV, Sabino AA, de Fatima A, de Resende MA (2008) Curcumin as a promising antifungal of clinical interest. J Antimicrob Chemother 63:337–339. https://doi.org/10.1093/jac/dkn488
dos Santos PDF, Francisco CRL, Coqueiro A, Leimann FV, Pinela J, Calhelha RC, Porto Ineu R, Ferreira ICFR, Bona E, Gonçalves OH (2019) The nanoencapsulation of curcuminoids extracted from Curcuma longa L. and an evaluation of their cytotoxic, enzymatic, antioxidant and anti-inflammatory activities. Food Funct 10:573–582. https://doi.org/10.1039/C8FO02431F
Keihanian F, Saeidinia A, Bagheri RK, Johnston TP, Sahebkar A (2018) Curcumin, hemostasis, thrombosis, and coagulation. J Cell Physiol 233:4497–4511. https://doi.org/10.1002/jcp.26249
Borges-Walmsley MI, Chen D, Shu X, Walmsley AR (2002) The pathobiology of Paracoccidioides brasiliensis. Trends Microbiol 10:80–87
Benard G (2008) An overview of the immunopathology of human paracoccidioidomycosis. Mycopathologia 165:209–221. https://doi.org/10.1007/s11046-007-9065-0
Harmsen S, McLaren AC, Pauken C, McLemore R (2011) Amphotericin B is cytotoxic at locally delivered concentrations. Clinical Orthopaed Related Res 469:3016–3021. https://doi.org/10.1007/s11999-011-1890-2
Laniado-Laborín R, Cabrales-Vargas MN (2009) Amphotericin B: side effects and toxicity. Rev Iberoam Micol 26:223–227. https://doi.org/10.1016/j.riam.2009.06.003
Spitzer M, Robbins N, Wright GD (2017) Combinatorial strategies for combating invasive fungal infections. Virulence 8:169–185. https://doi.org/10.1080/21505594.2016.1196300
Sharma M, Manoharlal R, Negi AS, Prasad R (2010) Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis: curcumin is synergistic to antifungals in Candida. FEMS Yeast Research no-no . https://doi.org/10.1111/j.1567-1364.2010.00637.x
Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, Aggarwal BB (2017) Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases: Curcumin: from kitchen to clinic. Br J Pharmacol 174:1325–1348. https://doi.org/10.1111/bph.13621
Sharma M, Manoharlal R, Puri N, Prasad R (2010) Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci Rep 30:391–404. https://doi.org/10.1042/BSR20090151
Dao TT, Sehgal P, Tung TT, Møller JV, Nielsen J, Palmgren M, Christensen SB, Fuglsang AT (2016) Demethoxycurcumin is a potent inhibitor of P-type ATPases from diverse kingdoms of life. PLoS ONE 11:e0163260 . https://doi.org/10.1371/journal.pone.0163260
Neelofar K, Shreaz S, Rimple B, Muralidhar S, Nikhat M, Khan LA (2011) Curcumin as a promising anticandidal of clinical interest. Can J Microbiol 57:204–210. https://doi.org/10.1139/W10-117
Kumar A, Dhamgaye S, Maurya IK, Singh A, Sharma M, Prasad R (2014) Curcumin targets cell wall integrity via calcineurin-mediated signaling in Candida albicans. Antimicrob Agents Chemother 58:167–175. https://doi.org/10.1128/AAC.01385-13
Gallis HA, Drew RH, Pickard WW (1990) Amphotericin B: 30 years of clinical experience. Rev Infect Dis 12:308–329
Baginski M, Czub J, Sternal K (2006) Interaction of amphotericin B and its selected derivatives with membranes: molecular modeling studies. Chem Rec 6:320–332. https://doi.org/10.1002/tcr.20096
Yoshida Y, Aoyama Y (1991) Sterol 14 alpha-demethylase and its inhibition: structural considerations on interaction of azole antifungal agents with lanosterol 14 alpha-demethylase (P-450(14DM)) of yeast. Biochem Soc Trans 19:778–782
Catalán M, Montejo JC (2006) Antifúngicos sistémicos. Farmacodinamia y farmacocinética. Rev Iberoam Micol 23:39–49. https://doi.org/10.1016/S1130-1406(06)70012-2
Mishra J, Dey A, Singh N, Somvanshi R, Singh S (2013) Evaluation of toxicity & therapeutic efficacy of a new liposomal formulation of amphotericin B in a mouse model. Indian J Med Res 137:767–776
Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K (2015) Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 10:e0121313. https://doi.org/10.1371/journal.pone.0121313
Hung W-C, Chen F-Y, Lee C-C, Sun Y, Lee M-T, Huang HW (2008) Membrane-thinning effect of curcumin. Biophys J 94:4331–4338. https://doi.org/10.1529/biophysj.107.126888
Chen C, Long L, Zhang F, Chen Q, Chen C, Yu X, Liu Q, Bao J, Long Z (2018) Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 13:e0194284. https://doi.org/10.1371/journal.pone.0194284
Ferreira FD, Mossini SAG, Ferreira FMD, Arrotéia CC, da Costa CL, Nakamura CV, Machinski Junior M (2013) The inhibitory effects of Curcuma longa L. essential oil and curcumin on Aspergillus flavus link growth and morphology. Sci World J 2013:1–6. https://doi.org/10.1155/2013/343804
Aloy P, Ceulemans H, Stark A, Russell RB (2003) The relationship between sequence and interaction divergence in proteins. J Mol Biol 332:989–998. https://doi.org/10.1016/j.jmb.2003.07.006
Tian W, Skolnick J (2003) How well is enzyme function conserved as a function of pairwise sequence identity? J Mol Biol 333:863–882. https://doi.org/10.1016/j.jmb.2003.08.057
Parente-Rocha JA, Parente AFA, Baeza LC, Bonfim SMRC, Hernandez O, McEwen JG, Bailão AM, Taborda CP, Borges CL, Soares CM de A (2015) Macrophage interaction with Paracoccidioides brasiliensis yeast cells modulates fungal metabolism and generates a response to oxidative stress. PLoS ONE 10:e0137619. https://doi.org/10.1371/journal.pone.0137619
Campos EG, Jesuino RSA, Dantas A da S, Brígido M de M, Felipe MSS (2005) Oxidative stress response in Paracoccidioides brasiliensis. Genet Mol Res 4:409–429
Al-Asmari F, Mereddy R, Sultanbawa Y (2017) A novel photosensitization treatment for the inactivation of fungal spores and cells mediated by curcumin. J Photochem Photobiol, B 173:301–306. https://doi.org/10.1016/j.jphotobiol.2017.06.009
do Carmo Silva L, Tamayo Ossa DP, Castro SV da C, Bringel Pires L, Alves de Oliveira CM, Conceição da Silva C, Coelho NP, Bailão AM, Parente-Rocha JA, Soares CM de A, Ruiz OH, Ochoa JGM, Pereira M (2015) Transcriptome profile of the response of Paracoccidioides spp. to a camphene thiosemicarbazide derivative. PLOS ONE 10:e0130703. https://doi.org/10.1371/journal.pone.0130703
Ahn S, Jung J, Jang I-A, Madsen EL, Park W (2016) Role of glyoxylate shunt in oxidative stress response. J Biol Chem 291:11928–11938. https://doi.org/10.1074/jbc.M115.708149
Costa M, Borges CL, Bailao AM, Meirelles GV, Mendonca YA, Dantas SFIM, de Faria FP, Felipe MSS, Molinari-Madlum EEWI, Mendes-Giannini MJS, Fiuza RB, Martins WS, Pereira M, Soares CMA (2007) Transcriptome profiling of Paracoccidioides brasiliensis yeast-phase cells recovered from infected mice brings new insights into fungal response upon host interaction. Microbiology 153:4194–4207. https://doi.org/10.1099/mic.0.2007/009332-0
Bastos KP, Bailão AM, Borges CL, Faria FP, Felipe MSS, Silva MG, Martins WS, Fiúza RB, Pereira M, Soares CMA (2007) The transcriptome analysis of early morphogenesis in Paracoccidioides brasiliensis mycelium reveals novel and induced genes potentially associated to the dimorphic process. BMC Microbiol 7:29. https://doi.org/10.1186/1471-2180-7-29
Kaur A, Sharma P, Capalash N (2018) Curcumin alleviates persistence of Acinetobacter baumannii against colistin. Sci Rep 8:11029. https://doi.org/10.1038/s41598-018-29291-z
(2012) Synthesis and in vitro antimycobacterial and isocitrate lyase inhibition properties of novel 2-methoxy-2′-hydroxybenzanilides, their thioxo analogues and benzoxazoles. European J Med Chem 56:108–119 . https://doi.org/10.1016/j.ejmech.2012.08.016
Yanik T, Donaldson RP (2005) A protective association between catalase and isocitrate lyase in peroxisomes. Arch Biochem Biophys 435:243–252. https://doi.org/10.1016/j.abb.2004.12.017
de Arruda GD, Bailão AM, Vieira Rezende TC, Borges CL, de Oliveira MAP, Parente JA, de Almeida Soares CM (2013) Response to oxidative stress in Paracoccidioides yeast cells as determined by proteomic analysis. Microbes Infect 15:347–364. https://doi.org/10.1016/j.micinf.2012.12.002
Lima P de S, Casaletti L, Bailão AM, Vasconcelos ATR de, Fernandes G da R, Soares CM de A (2014) Transcriptional and proteomic responses to carbon starvation in Paracoccidioides. PLoS Neglected Tropical Diseases 8:e2855. https://doi.org/10.1371/journal.pntd.000285
Sandoval JM, Arenas FA, Vásquez CC (2011) Glucose-6-phosphate dehydrogenase protects Escherichia coli from tellurite-mediated oxidative stress. PLoS ONE 6:e25573. https://doi.org/10.1371/journal.pone.0025573
Lundberg BE, Wolf RE, Dinauer MC, Xu Y, Fang FC (1999) Glucose 6-phosphate dehydrogenase is required for Salmonella typhimurium virulence and resistance to reactive oxygen and nitrogen intermediates. Infect Immun 67:436–438. https://doi.org/10.1128/IAI.67.1.436-438.1999
Dantas AS, Andrade RV, de Carvalho MJ, Felipe MSS, Campos ÉG (2008) Oxidative stress response in Paracoccidioides brasiliensis: assessing catalase and cytochrome c peroxidase. Mycol Res 112:747–756. https://doi.org/10.1016/j.mycres.2007.11.018
Funding
This work was performed at Universidade Federal de Goiás supported by MCTI/CNPq (Ministério da Ciência e Tecnologia/Conselho Nacional de Desenvolvimento Científico e Tecnológico), FNDCT (Fundo Nacional de Desenvolvimento Científico e Tecnológico), FAPEG (Fundação de Amparo à Pesquisa do Estado de Goiás), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/ Finance Code 001), FINEP (Financiadora de Estudos e Projetos), PRONEX (Programa de Apoio a Núcleos de Excelência), and INCT-IPH (Instituto Nacional de Ciência e Tecnologia de Estratégias de Interação Patógeno-Hospedeiro). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Each author has contributed significantly to this work. O.B.R., L.C.S., and M.A.B.C.J. performed the biological assays. A.A.O. performed the in silico assay. All authors contributed to the manuscript writing. All authors have given approval for the final version of the manuscript. O.B.R. and L.C.S. share the first authorship.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Carlos Pelleschi Taborda
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rocha, O.B., do Carmo Silva, L., de Carvalho Júnior, M.A.B. et al. In vitro and in silico analysis reveals antifungal activity and potential targets of curcumin on Paracoccidioides spp.. Braz J Microbiol 52, 1897–1911 (2021). https://doi.org/10.1007/s42770-021-00548-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00548-6