Abstract
Forest fragmentation can reduce population size, affecting the plant species and their biological interactions. We evaluated the effects of fragmentation on Ficus adhatodifolia Schott ex Spreng population, aiming to answer two questions: (1) is population size sufficient to maintain reproductive fig trees throughout the year? (2) Is the mean seed production per syconium higher in the larger forest fragments? Fifty-six trees of F. adhatodifolia were visited in a year, in five fragments and one urban area in southern Brazil. Mature syconia were collected and the seed and wasp production between areas were compared. Considering all forest remnants, we recorded syconia production throughout the year, but no fragment exhibited crop production in all months. The pollinator wasps Tetrapus sp. were found in almost all syconium, thus, indicating that Ficus-pollinator mutualism was not lost. Nevertheless, smaller fragments were not capable of maintaining reproductive individuals throughout the year, therefore, requiring pollinator wasps from other fragments. The size of fragments did not influence seed production [LRT P value = 0.1; LR stat (χ2) = 4.64; n = 370]. Factors such as Ficus resilience and pollinating wasp migration may have contributed to the support of mutualism so far. Therefore, in highly fragmented landscapes such as the study region, the conservation of all forest remnants is essential to mutualistic interaction preservation. This procedure combined with the restoration of degraded areas determined by law are urgent and necessary for the conservation of regional biodiversity.

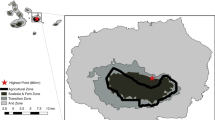
Source: Quantum GIS 2.14 and Google Earth (colour figure online)



Similar content being viewed by others
References
Aguiar LM, Reis NR, Ludwig G, Rocha VJ (2003) Dieta, área de vida, vocalizações e estimativas populacionais de Alouatta guariba em um remanescente florestal no norte do estado do Paraná [Diet, living area, vocalization and populational estimate of Alouatta guariba in a forest remaining from north of Parana state]. Neotrop Primates 11:78–86
Ahmed S, Compton SG, Butlin RK, Gilmartin PM (2009) Wind-borne insects mediate directional pollen transfer between desert fig trees 160 km apart. PNAS 106:20342–20347
Anstett MC, Kjellberg F, Bronstein JL (1996) Waiting for wasps: consequences for the pollination dynamics of Ficus pertusa L. J Biogeogr 23:459–466
Baijnath H, Ramcharun S (1983) Aspects of pollination and floral development in Ficus capensis Thunb. (Moraceae). Bothalia 14:883–888
Banack SA, Horn MH, Gawlicka A (2002) Disperser vs. establishment-limited distribution of a riparian fig tree (Ficus insipida) in a Costa Rican tropical rain forest. Biotropica 34:232–243
Bawa KS (1990) Plant-pollinator interactions in tropical rain forests. Annu Rev Ecol Syst 21:399–422
Berg CC (2006) The subdivision of Ficus subgenus Pharmacosycea section Pharmacosycea (Moraceae). Blumea 51:147–151
Berg CC, Villavicencio X (2004) Taxonomic studies on Ficus (Moraceae) in the West Indies, extra-Amazonian, Brazil, and Bolivia. Ilicifolia 4, University of Bergen, Bergen
Berg CC, Wiebes JT (1992) African fig trees and fig wasps. Koninklijke Nederlandse Akademie van Wetenschappen, Amsterdam
Bianchini E, Pimenta JA, Santos FAM (2001) Spatial and temporal variation in the canopy cover in a tropical semi-deciduous forest. Braz Arch Biol Technol 44:269–276
Bianchini E, Popolo RS, Dias MC, Pimenta JA (2003) Diversidade e estrutura de espécies arbóreas em área alagável do município de Londrina, sul do Brasil [Diversity and structure of arboreal species in wetlands from Londrina county, south of Brazil]. Acta Bot Bras 17:405–419
Bianchini E, Emmerick JM, Messetti AVL, Pimenta JA (2015) Phenology of two Ficus species in seasonal semi-deciduous forest in Southern Brazil. Braz J Biol 75:206–214
Bouček Z (1993) The genera of chalcidoid wasps from Ficus fruit in the new world. J Nat Hist 27:173–217
Bronstein JL (1988) Predators of fig wasps. Biotropica 20:215–219
Bronstein JL (1989) A mutualism at the edge of its range. Experientia 45:622–637
Bronstein JL (1992) Seed predators as mutualists: ecology and evolution of the fig/pollinator interaction. In: Bernays E (ed) Insect–plant interactions IV. CRC Press Inc., Boca Raton, pp 1–44
Bruna EM, Nardy O, Strauss SY, Harrison S (2002) Experimental assessment of Heliconia acuminate growth in a fragmented Amazonian landscape. J Ecol 90:639–649
Capinera JL (2008) Encyclopedia of enthomology. Springer Science, Dordrecht
Carauta JPP, Diaz BE (2002) Figueiras no Brasil [Fig trees in Brazil]. Editora UFRJ, Rio de Janeiro
Cardona W, Kattan G, Ulloa PC (2013) Non-pollinating fig wasps decrease pollinator and seed production in Ficus andicola (Moraceae). Biotropica 45:203–212
Chapin FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, Mack MC (2000) Consequences of changing biodiversity. Nature 405:234–242
Chen Y-R, Wu W-J, Chou L-S (2004) Synchronization of fig (Ficus microcarpa L.) abundance and pollinator (Eupristina verticillata: Agaoninae) population dynamics in northern Taiwan. J Nat Taiwan Mus 57:23–35
Compton SG, Robertson HG (1988) Complex interactions between mutualisms: ants tending homopterans protect fig seeds and pollinators. Ecology 69:1302–1305
David JP, Murugan BS, Manakadan R (2012) Seasonality in fruiting of fig and non-fig species in a tropical dry evergreen forest in Sriharikota Island, southern India. Trop Ecol 53:1–13
Dejean A, Bourgoin T, Gibernau M (1997) Ant species that protect figs against other ants: result of territoriality induced by a mutualistic homopteran. Ecoscience 4:446–453
Demétrio CGB, Hinde J, Moral RA (2014) Models for overdispersed data in entomology. In: Ferreira CP, Godoy WAC (eds) Ecological modelling applied to entomology. Springer, Berlin, pp 219–259
Dias MC, Vieira AOS, Paiva MRC (2002) Florística e fitossociologia das espécies arbóreas das florestas da bacia do rio Tibagi [Floristic and phytosociology of the forests arboreal species from Tibagi river basin]. In: Medri ME, Bianchini E, Shibatta AO, Pimenta JA (eds) A bacia do rio Tibagi [The Tibagi river basin]. Edição dos Editores, Londrina, pp 109–124
Elias LG, Farache FHA, Pereira RAS (2007) Efeito de vespas não-polinizadoras sobre o mutualismo Ficus-vespas de figos [Efect of non-pollinating wasps about Ficus-fig wasps mutualism]. Iheringia Ser Zool 97:253–256
Figueroa CC, Niemeyer HM, Cabrera-Brandt M, Briones LM, Lavandero B, Zuniga-Reinoso A, Ramirez CC (2018) Forest fragmentation may endanger a plant-insect interaction: the case of the highly specialist native aphid Neuquenaphis staryi in Chile. Insect Conserv Diver 11:352–362
Fournier LA (1974) Un método cuantitativo para la medición de características fenológicas en árboles [A quantitative method for measurement of phenological characteristics in trees]. Turrialba 24:422–423
Galil J, Eisikowitch D (1968) On the pollination ecology of Ficus sycomorus in east Africa. Ecology 49:259–269
Harris F, Johnson SD (2004) The consequences of habitat fragmentation for plant–pollinator mutualisms. Int J Trop Insect Sci 24:29–43
Harrison RD (2007) Maintenance of specificity in an isolated fig. Biotropica 39:275–277
Heer K, Kalko EKV, Albrecht L, Garcia-Villacorta R, Staeps FC, Herre EA, Dick WC (2015) Spatial scales of genetic structure in free-standing and strangler figs (Ficus, Moraceae) inhabiting neotropical forests. PLoS One 10:e0133581
Herre EA (1996) An overview of studies on a community of Panamanian figs. J Biogeogr 23:593–607
IBGE-Instituto Brasileiro de Geografia e Estatística (2012) Manual técnico da vegetação brasileira [Technical manual of Brazilian vegetation]. IBGE, Rio de Janeiro
Janzen D (1979) How many parents do the wasps from a fig have? Biotropica 11:127–129
Jevanandam N, Goh GR, Corlett RT (2013) Climate warming and the potential extinction of fig wasps, the obligate pollinators of figs. Biol Lett 9:1–4
Kearns CA, Inouye DW, Waser N (1998) Endangered mutualisms: the conservation of plant–pollinator interactions. Annu Rev Ecol Syst 29:83–112
Korine C, Kalko EKV, Herre EA (2000) Fruit characteristics and factors affecting fruit removal in a Panamanian community of strangler figs. Oecologia 123:560–568
Laurindo RS, Novaes RLM, Vizentin-Bugoni J, Gregorin R (2019) The effects of habitat loss on bat-fruit networks. Biodivers Conserv 28:589–601
Mackay KD, Gross CL (2019) Climate change threatens a fig-frugivore mutualism at its drier, western range margin. Proc Linn Soc N S W 141:1–17
Mawdsley NA, Compton SG, Whittaker RJ (1998) Population persistence, pollination mutualisms, and figs in fragmented tropical landscapes. Conserv Biol 12:1416–1420
Mendonça FA, Danni-Oliveira IM (2002) Dinâmica atmosférica e tipos climáticos predominantes da bacia do rio Tibagi [Atmospheric dynamics and prevailing climatic types of Tibagi river basin]. In: Medri ME, Bianchini E, Shibatta AO, Pimenta JA (eds) A bacia do Rio Tibagi [The Tibagi river basin]. Edição dos Editores, Londrina, pp 63–66
Milton K (1991) Leaf change and fruit production in six neotropical Moraceae species. J Ecol 79:1–26
Nason JD, Herre EA, Hamrick JL (1998) The breeding structure of a tropical keystone plant resource. Nature 391:685–687
Nazareno AG, Carvalho D (2009) What the reasons for no inbreeding and high genetic diversity of the neotropical fig tree Ficus arpazusa? Conserv Genet 10:1789–1793
Pardini R, Bueno AA, Gardner TA, Prado PI, Metzger JP (2010) Beyond the fragmentation threshold hypothesis: regime shifts in biodiversity across fragmented landscapes. PLoS One 5:0013666
Peng Y-Q, Compton SG, Yang D-R (2010) The reproductive success of Ficus altissima and its pollinator in a strongly seasonal environment: Xishuangbanna, Southwestern China. Plant Ecol 209:227–236
Pereira RAS, Semir J, Menezes AO Jr (2000) Pollination and other biotic interactions in figs of Ficus eximia Schott (Moraceae). Rev Bras Bot 23:217–224
Pereira RAS, Rodrigues E, Menezes AO Jr (2007) Phenological patterns of Ficus citrifolia (Moraceae) in a seasonal humid-subtropical region in Southern Brazil. Plant Ecol 188:265–275
Pryanga A (2004) Spatial dynamics of mutualistic interactions. J Anim Ecol 73:128–142
Rasplus JY, Soldati L (2006) Familia Agaonidae [Agaonidae family]. In: Fernández F, Sharkey MJ (eds) Introducción a los hymenoptera de la región neotropical [Introduction to hymenoptera from neotropical region]. Sociedad Colombiana de Entomologia & Universidad Nacional de Colômbia, Bogotá, pp 683–698
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153
Ricklefs RE (2010) A economia da natureza [The nature’s economy], 6a edn. Guanabara Koogan, Rio de Janeiro
Schatz B, Hossaert-McKey M (2003) Interactions of the ant Crematogaster scutellaris with the fig/fig wasp mutualism. Ecol Entomol 28:359–368
Schatz B, Kjellberg F, Nyawa S, Hossaert-McKey M (2008) Fig wasps: a staple food for ants on Ficus. Biotropica 40:190–195
Silva FC, Soares-Silva LH (2000) Arboreal flora of the Godoy Forest State Park, Londrina, PR, Brazil. Edinb J Bot 57:107–120
Tabarelli M, Silva JMC, Gascon C (2004) Forest fragmentation, synergisms and the impoverishment of Neotropical forests. Biodivers Conserv 13:1419–1425
Vicente RF (2006) O Parque Estadual Mata dos Godoy [The Godoy forest state park]. In: Torezan JMD (ed) Ecologia do Parque Estadual Mata dos Godoy [Ecology of Godoy forest state park]. Itedes, Londrina, pp 13–18
Walter DE (2000) First record of a fig mite from the Australian region: Paratarsonemella giblindavisisp.n. (Acari: Tarsonemidae). Aust J Entomol 39:229–232
Wang R-W, Yang C-Y, Zhao G-F, Yang J-X (2005) Fragmentation effects on diversity of wasp community and its impact on fig/fig wasp interaction in Ficus racemosa L. J Integr Plant Biol 47:20–26
Wang R-W, Dunn DW, Sun BF (2014) Discriminative host sanctions in a fig-wasp mutualism. Ecology 95:1384–1393
Weiblen GD (2002) How to be a fig wasp. Annu Rev Entomol 47:299–330
West SA, Herre EA, Windsor DM, Green PRS (1996) The ecology and evolution of the New World non-pollinating fig wasp communities. J Biogeogr 23:447–458
Acknowledgements
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Fundação Araucária for the PELD-MANP Grant (process number 441540/2016-3). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Brazil—Finance Code 001 (research grants and fellowship to LFFP). The authors also thank all the help received from Laboratório de Biologia Reprodutiva de Ficus, from Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto—USP, especially to Rodrigo A. S. Pereira and Fernando Farache.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pol, L.F.F., Pires, H.H., da Silva Ribeiro, J.E.L. et al. Effects of forest fragmentation on Ficus adhatodifolia Schott ex Spreng phenology and on its interactions with wasps. Trop Ecol 60, 462–471 (2019). https://doi.org/10.1007/s42965-019-00049-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-019-00049-6