Abstract
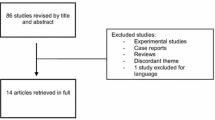
Dienogest (DNG) is a progestin with highly selective progesterone activity and known to be effective in the treatment of endometriosis. This prospective cohort study in patients who had been treated with DNG 2 mg (Visanne®) for endometriosis was conducted to assess the safety and effectiveness of DNG in a large Korean cohort. This study included 3356 patients with endometriosis from 73 centers in Korea. All patients were treated with DNG 2 mg daily and were followed up for at least 6 months after initial visit. Any adverse events were recorded including severity, onset/closing date, outcomes, treatments, and the causality with DNG. Effectiveness of DNG was measured by changes in visual analogue scale (VAS) from baseline at the end of follow-up. The mean age of the subjects was 34.96 years, and the mean duration of treatment was 285.44 days. Incidence of adverse drug reaction (ADR) was 13.27% (413/3113). The most frequently reported ADR were “abnormal uterine bleeding” 4.14% (129/3113), “increased weight” 2.57% (80/3113), and “headache” 1.22% (38/3113). The number of patients (%) with favorable bleeding patterns was observed to increase as the duration of treatment increases. Amenorrhea was observed in 29.63%, 41.25%, 46.26%, and 53.20% of patients at 3 months, 6 months, 12 months, and more than 12 months follow-up period, respectively. The mean (±SD) VAS change from baseline at the last follow-up visit was −28.19 ± 28.39 mm (P value < 0.0001). This large cohort study confirms, in routine clinical practice, that DNG is safe and effective for treatment of endometriosis.




Similar content being viewed by others
References
Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–704.
Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594–600.
Sinaii N, Cleary SD, Younes N, Ballweg ML, Stratton P. Treatment utilization for endometriosis symptoms: a cross-sectional survey study of lifetime experience. Fertil Steril. 2007;87(6):1277–86.
Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9.
Sinaii N, Plumb K, Cotton L, Lambert A, Kennedy S, Zondervan K, et al. Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil Steril. 2008;89(3):538–45.
Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--part 1. BJOG. 2008;115(11):1382–91.
Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, et al. The social and psychological impact of endometriosis on women's lives: a critical narrative review. Hum Reprod Update. 2013;19(6):625–39.
Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, de Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–12.
Fadhlaoui A, de la Jolinière JB, Feki A. Endometriosis and infertility: how and when to treat? Front Surg. 2014;1(24).
Fuldeore MJ, Soliman AM. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: National Estimates from a cross-sectional survey of 59,411 women. Gynecol Obstet Investig. 2017;82(5):453–61.
Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34–41.
Eisenberg VH, Weil C, Chodick G, Shalev V. Epidemiology of endometriosis: a large population-based database study from a healthcare provider with 2 million members. BJOG. 2018;125(1):55–62.
Zanelotti A, Decherney AH. Surgery and endometriosis. Clin Obstet Gynecol. 2017;60(3):477–84.
Endometriosis: diagnosis and management. National Institute for Health Care and Excellence (NICE). https://www.nice.org.uk/guidance/ng73. Published September 2017. Accessed September 2018.
Leyland N, Casper R, Laberge P, Singh SS, SOGC. Endometriosis: diagnosis and management. J Obstet Gynaecol Can. 2010;32(7 Suppl 2):S1–32.
Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril. 2004;82(4):878–84.
Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15(4):441–61.
Kondo W, Bourdel N, Tamburro S, Cavoli D, Jardon K, Rabischong B, et al. Complications after surgery for deeply infiltrating pelvic endometriosis. BJOG. 2011;118(3):292–8.
Prentice A, Deary AJ, Bland E. Progestogens and anti-progestogens for pain associated with endometriosis. Cochrane Database Syst Rev. 2000;2:CD002122.
Prentice A, Deary AJ, Goldbeck-Wood S, Farquhar C, Smith SK. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst Rev. 2007;2:CD000346.
Oettel M, Carol W, Elger W. A 19-norprogestin without 17a-ethinyl group II: dienogest from a pharmacodynamic point of view. Drugs Today. 1995;31:517–36.
Sasagawa S, Shimizu Y, Kami H, Takeuchi T, Mita S, Imada K, et al. Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profile. Steroids. 2008;73(2):222–31.
Strowitzki T, Faustmann T, Gerlinger C, Schumacher U, Ahlers C, Seitz C. Safety and tolerability of dienogest in endometriosis: pooled analysis from the European clinical study program. Int J Women's Health. 2015;7:393–401.
Köhler G, Faustmann TA, Gerlinger C, Seitz C, Mueck AO. A dose-ranging study to determine the efficacy and safety of 1, 2, and 4 mg of dienogest daily for endometriosis. Int J Gynaecol Obstet. 2010;108(1):21–5.
Strowitzki T, Faustmann T, Gerlinger C, Seitz C. Dienogest in the treatment of endometriosis-associated pelvic pain: a 12-week, randomized, double-blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):193–8.
Strowitzki T, Marr J, Gerlinger C, Faustmann T, Seitz C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010;25(3):633–41.
Petraglia F, Hornung D, Seitz C, Faustmann T, Gerlinger C, Luisi S, et al. Reduced pelvic pain in women with endometriosis: efficacy of long-term dienogest treatment. Arch Gynecol Obstet. 2012;285(1):167–73.
Lang J, Yu Q, Zhang S, et al. Dienogest for treatment of endometriosis in Chinese women: a placebo-controlled, randomized, double-blind phase 3 study. J Women's Health (Larchmt). 2018;27(2):148–55.
Yu Q, Zhang S, Li H, et al. Dienogest for treatment of endometriosis in women: a 28-week, open-label, extension study. J Women's Health (Larchmt). 2019;28(2):170–7.
American Society For Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–21.
Fraser IS, Critchley HO, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):383–90.
Gerlinger C, Schumacher U, Faustmann T, Colligs A, Schmitz H, Seitz C. Defining a minimal clinically important difference for endometriosis associated pelvic pain measured on a visual analog scale: analysis of two placebo controlled, randomized trials. Health Qual Life Outcomes. 2010;8:138.
Chudnoff SG, Nichols JE Jr, Levie M. Hysteroscopic essure inserts for permanent contraception: extended follow-up results of a phase III multicenter international study. J Minim Invasive Gynecol. 2015;22(6):951–60.
Guy W. ECDEU Assessment Manual for Psychopharmacology (028 clinical global impressions [CGI]). Rockville: National Institutes of Health; 1976. p. 218–22.
Heinemann K, Imthurn B, Marions L, Faustmann T. VIPOS: a large non-interventional study examining the safety of dienogest and other hormonal treatments for endometriosis in real-world clinical practice. Poster presented at: the Society of Endometriosis and Uterine Disorders; 2018; Florence, Italy.
Imthurn B, Marions L, Möhner S, Becker K, Faustmann T, Heinemann K. Baseline patient and disease characteristics of >27,000 women with endometriosis enrolled in the VIPOS study (Abstract). Fertil Steril. 2018;110(4):e392.
Kim BK, Chu MK, Lee TG, Kim JM, Chung CS, Lee KS. Prevalence and impact of migraine and tension-type headache in Korea. J Clin Neurol. 2012;8(3):204–11.
Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193–210.
Allais G, Chiarle G, Sinigaglia S, Benedetto C. Menstrual migraine: a review of current and developing pharmacotherapies for women. Expert Opin Pharmacother. 2018;19(2):123–36.
Pavlović JM, Allshouse AA, Santoro NF, Crawford SL, Thurston RC, Neal-Perry GS, et al. Sex hormones in women with and without migraine: evidence of migraine-specific hormone profiles. Neurology. 2016;87(1):49–56.
Darney PD. The androgenicity of progestins. Am J Med. 1995;98(1A):104S–10S.
Park SY, Kim SH, Chae HD, Kim CH, Kang BM. Efficacy and safety of dienogest in patients with endometriosis: a single-center observational study over 12 months. Clin Exp Reprod Med. 2016;43(4):215–20.
Alomar MJ. Factors affecting the development of adverse drug reactions (review article). Saudi Pharm J. 2014;22(2):83–94.
Untersmayr E, Jensen AN, Walch K. Sex hormone allergy: clinical aspects, causes and therapeutic strategies - update and secondary publication. World Allergy Organ J. 2017;10(1):45.
Funding
Funding for this study was provided by Bayer Korea Ltd.
Editorial support was provided by Honorem, which was funded by Bayer Korea Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Cho, B., Roh, JW., Park, J. et al. Safety and Effectiveness of Dienogest (Visanne®) for Treatment of Endometriosis: A Large Prospective Cohort Study. Reprod. Sci. 27, 905–915 (2020). https://doi.org/10.1007/s43032-019-00094-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-019-00094-5