Abstract
Purpose
Given the challenges associated with managing progressive scoliosis in patients with cerebral palsy (CP), the purpose of this study was to evaluate deformity correction and HRQOL 5 years post-spinal fusion in CP patients who were skeletally immature at the time of surgical correction.
Methods
CP patients who underwent definitive fusion before age 11 with minimum 5-years follow-up from a prospective, multicenter registry were included. Preoperative, initial postoperative, and 5-years radiographic data were collected. Preoperative and 5-years demographic, surgical data, complications, and CPCHILD outcome scores were analyzed. Repeated measures ANOVA with Bonferroni adjustment were used to analyze radiographic measures. Paired t test was utilized to compare outcomes. Significance was set at p = 0.05.
Results
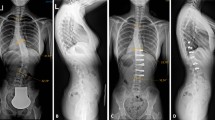
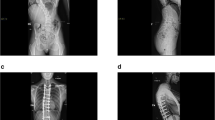
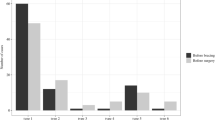
Twenty patients met inclusion—17 females, 3 males. The mean age was 9 (range 8–10) years. Eight-five percent had spastic CP with GMFCS Level V. Eighteen patients underwent posterior fusion; distal fixation was to the ilium in 80% and to L4-S1 in 20%. Significant correction of the primary curve (p ≤ 0.001) and pelvic obliquity (p ≤ 0.001) were obtained. From initial postoperative to 5-years follow-up there were no significant changes in major curve magnitude (p = 0.638), thoracic kyphosis (p = 0.09) or pelvic obliquity (p = 0.28). CPCHILD personal care, mobility, comfort, and total scores improved from preoperative to 5-years (p < 0.05). One patient needed a reoperation.
Conclusion
Surgical decision making for scoliosis in patients with CP can be difficult given the desire to maximize growth while minimizing adverse events. Performing a definitive fusion is a viable option that achieves good correction which remains stable 5 years postoperatively.
Similar content being viewed by others
References
Colver A, Fairhurst C, Pharoah PO (2014) Cerebral palsy. Lancet 383:1240–1249
Cloake T, Gardner A (2016) The management of scoliosis in children with cerebral palsy: a review. J Spine Surg 2:299–309
Persson-Bunke M, Hagglund G, Lauge-Pedersen H et al (2012) Scoliosis in a total population of children with cerebral palsy. Spine (Phila Pa 1976) 37:E708–E713
Hollenbeck SM, Yaszay B, Sponseller PD et al (2019) The Pros and Cons of operating early versus late in the progression of cerebral palsy scoliosis. Spine Deform 7:489–493
Comstock CP, Leach J, Wenger DR (1998) Scoliosis in total-body-involvement cerebral palsy. Analysis of surgical treatment and patient and caregiver satisfaction. Spine (Phila Pa 1976) 23:1412–1424 (discussion 24-5)
Sewell MD, Wallace C, Malagelada F et al (2015) Does spinal fusion and scoliosis correction improve activity and participation for children with GMFCS level 4 and 5 cerebral palsy? Medicine 94:e1907
Watanabe K, Lenke LG, Daubs MD et al (2009) Is spine deformity surgery in patients with spastic cerebral palsy truly beneficial?: a patient/parent evaluation. Spine (Phila Pa 1976) 34:2222–2232
Yaszay B, Sponseller PD, Shah SA et al (2017) Performing a definitive fusion in juvenile CP patients is a good surgical option. J Pediatr Orthop 37:e488–e491
Sitoula P, Holmes L Jr, Sees J et al (2016) The long-term outcome of early spine fusion for scoliosis in children with cerebral palsy. Clin Spine Surg 29:E406–E412
Smucker JD, Miller F (2001) Crankshaft effect after posterior spinal fusion and unit rod instrumentation in children with cerebral palsy. J Pediatr Orthop 21:108–112
Lonstein JE, Koop SE, Novachek TF et al (2012) Results and complications after spinal fusion for neuromuscular scoliosis in cerebral palsy and static encephalopathy using luque galveston instrumentation: experience in 93 patients. Spine (Phila Pa 1976) 37:583–591
Eguia F, Nhan DT, Shah SA et al (2020) Of major complication types, only deep infections after spinal fusion are associated with worse health-related outcomes in children with cerebral palsy. Spine (Phila Pa 1976) 45:993–999
Karol LA, Johnston C, Mladenov K et al (2008) Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am 90:1272–1281
Gilbert SR, Gilbert AC, Henderson RC (2004) Skeletal maturation in children with quadriplegic cerebral palsy. J Pediatr Orthop 24:292–297
Jain A, Sponseller PD, Shah SA et al (2016) Subclassification of GMFCS level-5 cerebral palsy as a predictor of complications and health-related quality of life after spinal arthrodesis. J Bone Joint Surg Am 98:1821–1828
Toovey R, Harvey A, Johnson M et al (2017) Outcomes after scoliosis surgery for children with cerebral palsy: a systematic review. Dev Med Child Neurol 59:690–698
Narayanan UG, Fehlings D, Weir S et al (2006) Initial development and validation of the Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD). Dev Med Child Neurol 48:804–812
Brooks JT, Sponseller PD (2016) What’s new in the management of neuromuscular scoliosis. J Pediatr Orthop 36:627–633
Miller DJ, Flynn JJM, Pasha S et al (2020) Improving health-related quality of life for patients with nonambulatory cerebral palsy: Who stands to gain from scoliosis surgery? J Pediatr Orthop 40:e186–e192
Miyanji F, Nasto LA, Sponseller PD et al (2018) Assessing the risk-benefit ratio of scoliosis surgery in cerebral palsy: surgery is worth it. J Bone Joint Surg Am 100:556–563
Sewell MD, Malagelada F, Wallace C et al (2016) A preliminary study to assess whether spinal fusion for scoliosis improves carer-assessed quality of life for children with GMFCS level IV or V cerebral palsy. J Pediatr Orthop 36:299–304
Bendon AA, George KA, Patel D (2016) Perioperative complications and outcomes in children with cerebral palsy undergoing scoliosis surgery. Paediatr Anaesth 26:970–975
Nishnianidze T, Bayhan IA, Abousamra O et al (2016) Factors predicting postoperative complications following spinal fusions in children with cerebral palsy scoliosis. Eur Spine J 25:627–634
Sponseller PD, Shah SA, Abel MF et al (2010) Infection rate after spine surgery in cerebral palsy is high and impairs results: multicenter analysis of risk factors and treatment. Clin Orthop Relat Res 468:711–716
McElroy MJ, Sponseller PD, Dattilo JR et al (2012) Growing rods for the treatment of scoliosis in children with cerebral palsy: a critical assessment. Spine (Phila Pa 1976) 37:E1504–E1510
Canavese F, Dimeglio A (2013) Normal and abnormal spine and thoracic cage development. World J Orthop 4:167–174
Dimeglio A, Canavese F (2012) The growing spine: how spinal deformities influence normal spine and thoracic cage growth. Eur Spine J 21:64–70
Acknowledgements
This study was supported in part by grants to the Setting Scoliosis Straight Foundation in support of Harms Study Group research from DePuy Synthes Spine, EOS imaging, Stryker Spine, Medtronic, NuVasive, Zimmer Biomet and the Food and Drug Administration.
Harms Study Group Investigators: Aaron Buckland, MD; Royal Children’s Hospital – Melbourne Australia; Amer Samdani, MD; Shriners Hospitals for Children—Philadelphia; Amit Jain, MD; Johns Hopkins Hospital; Baron Lonner, MD; Mount Sinai Hospital; Benjamin Roye, MD; Columbia University; Burt Yaszay, MD; Rady Children’s Hospital; Chris Reilly, MD; BC Children’s Hospital; Daniel Hedequist, MD; Boston Children’s Hospital; Daniel Sucato, MD; Texas Scottish Rite Hospital; David Clements, MD; Cooper Bone & Joint Institute New Jersey; Firoz Miyanji, MD; BC Children’s Hospital; Harry Shufflebarger, MD; Paley Orthopedic & Spine Institute; Jack Flynn, MD; Children’s Hospital of Philadelphia; John Asghar, MD; Paley Orthopedic & Spine Institute; Jean Marc Mac Thiong, MD; CHU Sainte-Justine; Joshua Pahys, MD; Shriners Hospitals for Children—Philadelphia; Juergen Harms, MD; Klinikum Karlsbad-Langensteinbach, Karlsbad; Keith Bachmann, MD; University of Virginia; Lawrence Lenke, MD; Columbia University; Lori Karol, MD; Children’s Hospital, Denver Colorado; Mark Abel, MD; University of Virginia; Mark Erickson, MD; Children’s Hospital, Denver Colorado; Michael Glotzbecker, MD; Rainbow Children’s Hospital, Cleveland; Michael Kelly, MD; Washington University; Michael Vitale, MD; Columbia University; Michelle Marks, PT, MA; Setting Scoliosis Straight Foundation; Munish Gupta, MD; Washington University; Nicholas Fletcher, MD; Emory University; Noelle Larson, MD; Mayo Clinic Rochester Minnesota; Patrick Cahill, MD; Children’s Hospital of Philadelphia; Paul Sponseller, MD; Johns Hopkins Hospital; Peter Gabos, MD: Nemours/Alfred I. duPont Hospital for Children; Peter Newton, MD; Rady Children’s Hospital; Peter Sturm, MD; Cincinnati Children’s Hospital; Randal Betz, MD; Institute for Spine & Scoliosis; Stefan Parent, MD: CHU Sainte-Justine; Stephen George, MD; Nicklaus Children's Hospital; Steven Hwang, MD; Shriners Hospitals for Children—Philadelphia; Suken Shah, MD; Nemours/Alfred I. duPont Hospital for Children; Sumeet Garg, MD; Children’s Hospital, Denver Colorado; Tom Errico, MD; Nicklaus Children's Hospital; Vidyadhar Upasani, MD; Rady Children’s Hospital.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Consortia
Contributions
BY, PDS, SAS, FM, AFS, RH, PON: conception or design of the work; or acquisition, analysis, or interpretation of data for the work; BY, PDS, SAS, FM, AFS, RH, PON: drafting or critically revising the work; BY, PDS, SAS, FM, AFS, RH, PON: final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
Roland Howard, MD: No Conflicts; Paul Sponseller, MD: During the Conduct of the Study: grants from Setting Scoliosis Straight Foundation. Outside the Submitted Work: personal fees from DePuy Synthes Spine. Suken A. Shah, MD: During the Conduct of the Study: grants from Setting Scoliosis Straight Foundation. Outside the Submitted Work: personal fees from DePuy Synthes Spine. Firoz Miyanji, MD: During the Conduct of the Study: grants from Setting Scoliosis Straight Foundation. Outside the Submitted Work: personal fees from Depuy Synthes Spine, personal fees from Stryker Spine, personal fees from Zimmer Biomet. Amer F. Samdani, MD: During the conduct of the study: grants from Setting Scoliosis Straight Foundation. Outside the submitted work: personal fees from DePuy Synthes Spine, personal fees from Ethicon, personal fees from Globus Medical, personal fees from Medical Device Business Services, personal fees from Mirus, personal fees from NuVasive, personal fees from Orthofix, personal fees from Stryker, personal fees from Zimmer Biomet. Peter O. Newton, MD: During the conduct of the study: grants from Setting Scoliosis Straight Foundation. Outside the submitted work: grants and other from Setting Scoliosis Straight Foundation, other from Rady Children's Specialists, grants, personal fees and non-financial support from DePuy Synthes Spine, grants and other from SRS, grants from EOS imaging, personal fees from Thieme Publishing, grants from NuVasive, other from Electrocore, personal fees from Cubist, other from International Pediatric Orthopedic Think Tank, grants, non-financial support and other from Orthopediatrics, grants, personal fees and non-financial support from Stryker/K2M, grants and non-financial support from Alphatech, grants from Mazor Robotics, personal fees from MiRus, personal fees from Globus Medical, personal fees from Pacira, from Scoliosis Research Society. In addition, Dr. Newton has a patent Anchoring systems and methods for correcting spinal deformities (8540754) with royalties paid to DePuy Synthes Spine, a patent Low profile spinal tethering systems (8123749) licensed to DePuy Spine, Inc., a patent Screw placement guide (7981117) licensed to DePuy Spine, Inc., a patent Compressor for use in minimally invasive surgery (7189244) licensed to DePuy Spine, Inc., and a patent Posterior spinal fixation pending to K2M. Burt Yaszay, MD: During the conduct of the study: grants from Setting Scoliosis Straight Foundation. Outside the submitted work: grants and personal fees from DePuy Synthes Spine, grants and personal fees from Nuvasive, personal fees from Medtronic, grants and personal fees from Orthopediatrics, grants and personal fees from K2M/Stryker, personal fees from Globus, grants from Setting Scoliosis Straight Foundation, personal fees from Biogen. In addition, Dr. Yaszay has a patent K2M/Stryker with royalties paid.
Ethical statement
IRB approval was obtained for this study and consent was obtained from all subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the Harms Study Group Investigators was processed under acknowledgements section.
Rights and permissions
About this article
Cite this article
Howard, R., Sponseller, P.D., Shah, S.A. et al. Definitive fusion for scoliosis in late juvenile cerebral palsy patients is durable at 5 years postoperatively. Spine Deform 10, 1423–1428 (2022). https://doi.org/10.1007/s43390-022-00530-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-022-00530-8