Abstract
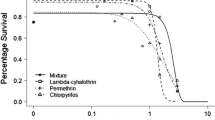
An important uncertainty often identified inecological risk assessment is the lack of ecologicalconnectivity between endpoints measured across themany levels of biological organization within anecosystem. In the present study, we address thisissue by quantitatively linking acetylcholinesterase(AChE) activity, a common biomarker of exposure toorganophosphorus (OP) insecticides, with endpoints athigher levels of biological organization in fish andinvertebrates, and to assess the utility of thisendpoint as a predictive biomarker of effects underfield conditions. In 1997, we conducted three fieldstudies in outdoor microcosms to assess binary andternary mixtures of chlorpyrifos (CLP),azinphos-methyl (AZM), and diazinon (DIA). The firststudy (14 days) used a regression design(concentration-response) to assess direct and indirectpopulation-level responses of zooplankton andphytoplankton to a binary mixture of DIA and CLP atnominal concentrations of 0.44–44.0 μg/L. A secondregression study (7 days) was conducted to assesslethality (organismal response) in fathead minnows(Pimephales promelas) to a ternary mixture ofthe three OP's at nominal concentrations of 50–1750 μg/L. An ANOVA study (7 days) was also conducted toassess lethality in fathead minnows. In this study,the concentrations of the components of the mixturewere determined from exposure and toxicitydistributions using a toxic equivalent (TE) approachand apportioned to achieve equipotent mixtures at80:10:10 ratios. The abundance of Cladocera declinedby close to 100% within 24 hours of application atthe four highest concentrations; consequently, AChEcould not be measured in these treatments. At the twolowest treatments, AChE activity exhibited aconcentration-dependent decline over the study period;however, AChE activity increased over the first 24hours of exposure, while abundance decreased. Infathead minnows, mortality was significantlycorrelated with brain AChE activity in both studies. The form of this relationship was remarkablyconsistent between studies, with a 50% reduction inAChE activity corresponding to 10–15% mortality anda 90% inhibition of AChE corresponding to 50%mortality. The results of these studies show thatAChE activity, a biochemical endpoint, can be used asa reliable biomarker of effect at the organismal leveland may be useful as a predictor of population-levelresponses in invertebrates.
Similar content being viewed by others
References
Attril, M.J. & M.H. Depledge, 1997. Community and population indicators of ecosystem health: targeting links between levels of biological organization. Aquat. Toxicol. 38: 183–197.
Bestari, K.T., R.D. Robinson, K.R. Solomon, T.S. Steele, K.E. Day & P.K. Sibley, 1998. Distribution and composition of polycyclic aromatic hydrocarbons within experimental microcosms treated with liquid creosote. Environ. Toxicol. Chem. 17: 2359–2368.
Bogdan, K.G. & J.J. Gilbert, 1982. Seasonal patterns of feeding by natural populations of Keratella, Polyarthra, and Bosmina: clearance rates, selectivity, and contributions to community grazing. Limnol. Oceanog. 27: 918–934.
Boudou, A. & F. Ribeyre, 1997. Aquatic ecotoxicology: From the ecosystem to the cellular and molecular levels. Environ. Health Perspec. 105: 21–35.
Bradford, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Brock, T.C.M., M. Van den Bogaert, A.R. Bos, S.W.F. van Breukelen, R. Reiche, J. Terwoert, R.E.M. Suykerbuyk & R.M.M. Roijackers, 1992. Fate and effects of Dursban 4E in indoor Elodea-dominated and macrophyte-free freshwater model ecosystems: II Secondary effects on community structure. Arch. Environ. Contam. Toxicol. 23: 391–409.
Cairns, J. Jr. & B.R. Niederlehner, 1995. Predictive Ecotoxicology. In: D.J. Hoffman, B.A. Barnett, G.A. Burton, Jr & J. Cairns Jr (eds), Ecotoxicology, Lewis Publishers, Boca Raton, FL, pp. 67–680.
Calow, P., 1994. Ecotoxicology: What are we trying to protect? Environ. Toxicol. Chem. 13: 1549.
Clements, W.H. & P.M. Kiffney, 1994. Assessing contaminant effects at higher levels of biological organization. Environ. Toxicol. Chem. 13: 357–359.
Cormier, S.M. & F.B. Daniel, 1994. Biomarkers: Taking the science forward. Environ. Toxicol. Chem. 13: 1011–1012.
Day, K.E., N.K. Kaushik & K.R. Solomon, 1987. Impact of fenvalerate on enclosed freshwater planktonic communities and on in situ rates of filtration on zooplankton. Can. J. Fish. Aquat. Sci. 44: 1714–1728.
Dell'Omo, G., Bryenton, R. & R.F. Shore, 1997. Effects of exposures to an organophosphate pesticide on behavior and acetylcholinesterase activity in the common shrew, Sorex araneus. Environ. Toxicol. Chem. 16: 272–276.
Ellman, G.L., K.D. Courtney, V. Andres, Jr. & R.M. Featherstone, 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7: 88–95.
Escartin, E. & C. Porte, 1997. The use of cholinesterase and carboxylesterase activities from Mytilus galloprovincialis in pollution monitoring. Environ. Toxicol. Chem. 16: 2090–2095.
Fornstrom, C.B., P.F. Landrum, C.P. Weisskopf & T.W. LaPoint, 1997. Effects of turbofos on juvenile red swamp crayfish (Procambarus clarki): Differetial routes of exposure. Environ. Toxicol. Chem. 16: 2514–2520.
Galgani, F. & G. Bocquene, 1991. Semi-automated colorimetric and enzymatic assays for aquatic organisms using microplate readers. Water Res. 25: 147–150.
Gibson, R.F., J.L. Ludke & D.E. Ferguson, 1969. Sources of error in the use of fish-brain acetylcholinesterase as a monitor for pollution. Bull. Environ. Contam. Toxicol. 4: 17.
Giddings, J.M., R.C. Biever, M.E. Annunziato & A.J. Hosmer 1996. Effects of diazinon on large outdoor pond microcosms. Environ. Toxicol. Chem. 15: 618–629.
Giesy, J.P., K.R. Solomon, J.R. Coats, K.R. Dixon, J.M. Giddings & E.E. Kenaga, 1999. Chlorpyrifos: Ecological risk assessment in North American aquatic environments. Rev. Environ. Contam. Toxicol. 160: 1–129.
Gilbert, J.J. & K.G. Bogdan, 1984. Rotifer grazing: in situ studies on selectivity and rates. In: D.G. Meyers & J.R. Stickler (eds), Trophic Iteractions within Aquatic Ecosystems, American Society for the Advancement of Science Selected Symposium 85. Boulder, CO, pp. 97–133.
Graney, R.L., J.H. Kennedy & J.H. Rodgers, Jr., 1994. Aquatic Mesocosm Studies in Ecological Risk Assessment, CRC Press, Inc. Boca Raton, FL, 723 pp.
Hill, I.R., F. Heimbach, P. Leeuwangh & P. Matthiessen, 1994. Freshwater Field Tests for Hazard Assessment of Chemicals, Lewis Publishers, Boca Raton, FL, 661 pp.
Ingersoll, C.G., T. Dillon & G.R. Biddinger, 1997. Ecological Risk Assessment of Contaminated Sediments, SETAC Press, Pensacola, FL, 389 pp.
Ingersoll, C.G. et al., 1997. Workgroup summary report on uncertainty evaluation of measurement endpoints used in sediment ecological risk assessment. In: C.G. Ingersoll, T. Dillon & G.R. Biddinger (eds), Ecological Risk Assessment of Contaminated Sediments, Setac Press, Pensacola, FL, pp. 297–352.
Jensen, C.S., L. Garsdal & E. Baatrup, 1997. Acetylcholinesterase inhibition and altered locomotor behavior in the carabid beetle Pterostichus cupreus. A linkage between biomarkers at two levels of biological complexity. Environ. Toxicol. Chem. 16: 1727–1732.
Kaushik, N.K., G.L. Stephenson, K.R. Solomon & K.E. Day, 1985. Impact of permethrin on zooplankton communities in limnocorrals. Can. J. Fish. Aquat. Sci. 42: 77–85.
Kersting, K. 1994. Functional endpoints in field testing. In: I.R. Hill, F. Heimbach, P. Leeuwangh & P. Matthiesson (eds), Freshwater Field Test for Hazard Assessment of Chemicals, Lewis Publishers, Baca Raton, FL, pp. 57–81.
Kumar, A. & J.C. Chapman, 1998. Profenofos toxicity to the eastern rainbow fish (Melanotaenia duboulayi). Environ. Toxicol. Chem. 17: 1799–1806.
McCarty, L.S. & M. Power, 1997. Environmental risk assessment within a decision-making framework. Environ. Toxicol. Chem. 16: 122–123.
Melancon, M.J., 1995. Bioindicators used in aquatic and terrestrial monitoring. In D.J. Hoffman, B.A. Barnett, G.A. Burton, Jr & J. Cairns Jr (eds), Ecotoxicology, Lewis Publishers, Boca Raton, FL, pp. 220–240.
Menzie, C.A. & J.S. Freshman, 1997. An assessment of the risk assessment paradigm for ecological risk assessment. Human Ecol. Risk Assess. 3: 853–892.
Power, M. & L.S. McCarty, 1996. Probabilistic risk assessment: Betting on its future. Human Ecol. Risk Assess. 2: 35–43.
Power, M. & L.S. McCarty, 1997. Fallacies in ecological risk assessment practices. Environ. Sci. Technol. 31: 370–375.
Richardson, G.M., 1996. Deterministic versus probabilistic risk assessment: Strengths and weaknesses in a regulatory context. Human and Ecol. Risk Assess. 2: 55–58.
Safe, S. 1990. Polychlorinated biphenyls (PCB's), dibenzo-pdioxins (PCDD's), dibenzofurans (PCDF's), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEF's). Crit. Rev. Toxicol. 21: 51–58.
Safe, S. 1998. Hazard and risk assessment of chemical mixtures using the toxic equivalency factor approach. Environ. Health Persp. 106: 1051–1055.
Sibley, P.K., D.A. Benoit & G.T. Ankley, 1997. The significance of growth in Chironomus tentans sediment toxicity tests: Relationship to reproduction and demographic endpoints. Environ. Toxicol. Chem. 16(2): 336–345.
Solomon, K.R., 1996. Overview of recent developments in ecotoxicological risk assessment. Risk Anal. 16: 627–633.
Solomon, K.R., D.B. Baker, R.P. Richards, K.R. Dixon, S.J. Klaine, T.W. LaPoint, R.J. Kendall, J.M. Giddings, J.P. Giesy, L.W. Hall, Jr. & W.M. Williams, 1996. Ecological risk assessment of atrazine in North American surface waters. Environ. Toxicol. Chem. 15: 31–76.
Solomon, K.R. & M.J. Chappel, 1998. Triazine herbicides: Ecological risk assessment in surface waters. In: L.G. Ballantine, J.E. McFarland & D.S. Hackett (eds), Triazine Herbicides: Risk Assessment, American Chemical Society Symposium Series 683, pp. 367–368.
Suter, G.W., 1993. Ecological Risk Assessment, Lewis Publishers, Chelsea, MI.
Tillet, D.E., K.R. Solomon, E.M. Mihaich, G. Cobb, L. Touart & T.J. Kubiak, 1998. Role of exposure assessment in characterizing risks of endocrine-disrupting substances to wildlife. In: R. Kendall, R. Dickerson, J. Giesy & W. Suk (eds), Principles and Processes for Evaluating Endocrine Disruption in Wildlife, SETAC Press, Pensacola, FL, pp. 39–68.
United States Environmental Protection Agency, 1992. Framework for ecological risk assessment, EPA-630/R-92/001, Washington, D.C.
Webber, E.C., W.G. Deutsch, D.R. Bayne & W.C. Seesock, 1992. Ecosystem-level testing of a synthetic pyrethroid insecticide in aquatic mesocosms. Environ. Toxicol. Chem. 11: 87–105.
Weiss, C.M., 1958. The determination of cholinesterase in the brain tissue of three species of fresh water fish and its inactivation in vivo. Ecology 39: 194.
Weiss, C.M., 1961. Physiological effect of organic phosphorus insecticides on several species of fish. Trans. Am. Fish. Soc. 90: 143.
Whiteside, M.C. & J.B. Williams, 1975. A new sampling technique for aquatic ecologists. Limnologie 19: 1534–1539.
Yasuno, M., J. Okita & S. Hatakeyama, 1982. Effects of temephos on the macrobenthos in a stream of Mt. Tsukuba. Japanese J. Ecol. 32: 29–38.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sibley, P., Chappel, M., George, T. et al. Integrating effects of stressors across levels of biological organization: examples using organophosphorus insecticide mixtures in field-level exposures. Journal of Aquatic Ecosystem Stress and Recovery 7, 117–130 (2000). https://doi.org/10.1023/A:1009967213300
Issue Date:
DOI: https://doi.org/10.1023/A:1009967213300