Abstract
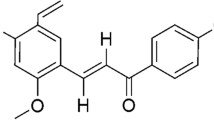
α-Hederin, a pentacyclic triterpene saponin isolated from the seeds of Nigella sativa, was recently reported to have potent in vivo antitumor activity against LL/2 (Lewis Lung carcinoma) in BDF1 mice. In this study we observed that α-hederin caused a dose- and time-dependent increase in apoptosis of murine leukemia P388 cells. In order to evaluate the possible mechanisms for apoptosis, the effects of α-hederin on intracellular thiol concentration, including reduced glutathione (GSH), and protein thiols, and the effects of pretreatment with N-acetlycysteine (NAC), a precursor of intracellular GSH synthesis, or buthionine sulfoxime (BSO), a specific inhibitor of intracellular GSH synthesis, on α-hederin-induced apoptosis were investigated. It was found that α-hederin rapidly depleted intracellular GSH and protein thiols prior to the occurrence of apoptosis. NAC significantly alleviated α-hederin-induced apoptosis, while BSO augmented α-hederin-induced apoptosis significantly. The depletion of cellular thiols observed after α-hederin treatment caused disruption of mitochondrial membrane potential (ΔΨm) and subsequently increased the production of reactive oxygen species (ROS) in P388 cells at an early time point. Bongkrekic acid (BA), a ligand of the mitochondrial adenine nucleotide translocator, and cyclosporin (CsA) attenuated the α-hederin-induced loss of ΔΨm, and ROS production. Thus, oxidative stress after α-hederin treatment is an important event in α-hederin-induced apoptosis. As observed in this study, permeability transition of mitochondrial membrane occurs after depletion of GSH and precedes a state of reactive oxygen species (ROS) generation. Further, we observed that α-hederin caused the release of cytochrome c from the mitochondria to cytosol, leading to caspase-3 activation. Our findings thus demonstrate that changes in intracellular thiols and redox status leading to perturbance of mitochondrial functions are important components in the mechanism of α-hederin-induced cell death.
Similar content being viewed by others
References
Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA: Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med 1: 1046-1051, 1995
Pisha E, Kim DSHL, Pezzuto JM: Synthesis of Betulinic acid derivatives with activity against human melanoma. Bioorg Med Chem Lett 8: 707-1712, 1998
Kim DSHL, Jeong H, Chai H: Preparation of amino acid conjugates of betulinic acid with activity against human melanoma. Bioorg Med Chem Lett 9: 1201-1204, 1999
Marciani DJ, Press JB, Reynolds RC, Pathak AK, Pathak V, Gundy LE, Farmer JT, Koratich MS, May RD: Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine 18: 3141-3151, 2000
Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K, Ma W, Georgiadis C, Laskin JD, Conney AH: Inhibition of skin tumorigensis by rosemary and its constituents carnesol and ursolic acid. Cancer Res 54: 701-708, 1994
Nishino H, Nishino A, Takayasu J, Hasegawa T, Iwashima A, Hirabayashi K, Iwata S, Shibata S: Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol-13-acetate by some oleanane-type triterpenoid compounds. Cancer Res 48: 5210-5215, 1988
Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, Xie QW, Nathan C, Gribble GW, Sporn MB: Triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res 58: 717-723, 1998
Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, Maue RA, Place AE, Porter DM, Spinella MJ, Williams CR, Wu G, Dannenberg AJ Flanders KC, Letterio JJ, Mangelsdorf DJ, Nathan CF, Nguyen L, Porter WW, Ren RF, Roberts AB, Roche NS, Subbaramaiah K, Sporn MB: A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res 59: 336-341, 1999
Fulda S, Scaffidi C, Susin SA, Krammer PH, Kroemer G, Peter ME, Debatin KM: Activation of mitochondria and release of mitochondrial apoptogenic factors by betulinic acid. J Biol Chem 273: 33942-33948, 1998
Wick W, Grimmel C, Wagenknecht B, Dichgans J, Weller M: Betulinic acid-induced apoptosis in glioma cells: A sequential requirement for new protein synthesis, formation of reactive oxygen species, and caspase processing. J Pharmacol Exp Ther 289: 1306-1312, 1999
Kim DK, Baek JH, Kang CM, Yoo MA, Sung JW, Chung HY, Kim ND, Choi YH, Lee SH, Kim KW: Apoptotic activity of ursolic acid may correlate with the inhibition of initiation of DNA replication. Int J Cancer 87: 629-636, 2000
Hostettmann, K: Saponins with molluscicidal activity. Helvetica Chemica Acta 63: 606-608, 1980
Danloy S, Quetin-Leclercq J, Coucke P, De Pauw-Gillet MC, Elias R, Balansard G, Angenot L, Bassleer R: Effects of α-hederin, a saponin extracted from Hedera helix, on cells cultured in vitro. Planta Med 60: 45-49, 1994
Swamy SMK, Tan BKH: Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella sativa seeds. J Ethnopharmacol 70: 1-7, 2000
Kumara SS, Huat BT: Extraction, isolation and characterization of antitumor principle, α-hederin, from the seeds of Nigella sativa. Planta Med 67: 29-32, 2001
Raff MC: Social controls on cell survival and cell death. Nature 356: 397-400, 1992
Isaacs JT: Role of programmed cell death in carcinogenesis. Environ Health Perspect 101: 27-34, 1993
Bursch W, Oberhammer F, Schulte-Hermann R: Cell death by apoptosis and its protective role against disease. Trends Pharmacol Sci 13: 245-251, 1992
Hall AG: The role of glutathione in the regulation of apoptosis. Eur J Clin Invest 9: 38-245, 1999
Ghibelli L, Rotilio CFG, Lafavia E, Coppola S, Colussi C, Civitareale, Ciriolo R: Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J 12: 479-486, 1998
Dai J, Weinberg RS, Waxman S, Jing Y: Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood 93: 268-277, 1999
Sato N, Iwata S, Kazuhiro N, Hori T, Mori K, Yodoi J: Thiol-mediated redox regulation of apoptosis. Possible roles of cellular thiols other than glutathione in T cell apoptosis. J Immunol 154: 3194-3203, 1995
Kroemer G, Zamzami N, Susin SA: Mitochondrial control of apoptosis. Immunol Today 18: 44-51, 1997
Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B: The mitochondrial permeability transition in cell death: A common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1366: 177-196, 1998
Crompton M: The mitochondrial permeability transition pore and its role in cell death. J Biochem 341: 233-249, 1999
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD: The release of cytochrome C from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 275: 1132-1136, 1997
Susin SA, Zamzami N, Castedo M: Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med 184: 1131-1141, 1996
Mujoo K, Haridas V, Hoffmann JJ, Wachter GA, Hutter LK, Lu Y, Blake ME, Jayatilake GS, Bailey D, Mills GB, Gutterman JU: Triterpenoid saponins from Acacia victoriae (Bentham) decrease tumor cell proliferation and induce apoptosis. Cancer Res 61: 5486-5490, 2001
Haridas V, Higuchi M, Jayatilake GS, Bailey D, Mujoo K, Blake ME, Arntzen CJ, Gutterman JU: Avicins: Triterpenoid saponins from Acacia victoriae (Bentham) induce apoptosis by mitochondrial perturbation. Proc Natl Acad Sci 98: 5821-5826, 2001
Geran RL, Greenberg NH, MacDonald MM, Schumacher AM, Abbott BJ: Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep 3: 1-103, 1972
Mosmann T: Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Meth 65: 55-63, 1983
Scudiero DA, Shoemaker RH, Paul KD, Monks A, Tierney S, Nofziger H, Currens MJ, Seniff D, Boyd MR: Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res 48: 4827-4833, 1988
Shao Y, Chin CK, Ho CT, Ma W, Garrison SA, Huang MT: Anti-tumor activity of the crude saponins obtained from asparagus. Cancer Lett 104: 31-36, 1996
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C: A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Meth 139: 271-279, 1991
Allen RT, Hunter WJ III, Agrawal DK: Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Meth 37: 215-228, 1997
Hissin PJ, Hilf R: A fluorometric method for determination of oxidized and reduced glutathione in tissue. Anal Biochem 74: 214-226, 1976
Albano E, Rundgren M, Harvison PJ, Nelson SD, Moldeus P: Mechanism of N-acetyl-p-benzoquinone imine cytotoxicity. Mol Pharmacol 28: 306-311, 1985
Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere JL, Petit PX, Kroemer G: Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med 181: 1661-1672, 1995
Peters TR, Tosk JM, Goulbourne EA: Lucigenin chemiluminescence as probe for measuring reactive oxygen species production in E. coli. Anal Biochem 186: 316-319, 1990
Ma Y, Ogino T, Kawabata T, Li J, Eguchi K, Okada S: Cupric nitrilotriacetate-induced apoptosis in HL-60 cells association with lipid peroxidation, release of cytochrome c from mitochondria, and activation of caspase-3. Free Radic Biol Med 27: 227-233, 1999
Wyllie AH, Kerr JFR, Currie AR: Cell death: The significance of apoptosis. Int Rev Cytol 68: 251-306, 1980
Bossy-wetzel E, Newmeyer DD, Green DR: Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J 17: 37-49, 1998
Uehara T, Kikuchi Y, Nomura Y: Caspase activation accompanying cytochrome c release from mitochondria in possibly involved in nitric oxide-induced neuronal apoptosis n SH-SY5Y cells. J Neurochem 72: 196-205, 1999
Baker MA, Hagner BA: Diamide induced shift in protein and glutathione thiol: Disulfide status delays DNA rejoining after X-irradiation of human cancer cells. Biochem Biophys Acta 1037: 39-47, 1990
Mba Gachou C, Laget M, Guiraud-Dauriac H, De Meo M, Elias R, Dumenil G: The protective activity of alpha-hederin against H2O2 genotoxicity in HepG2 cells by alkaline comet assay. Mutat Res 445: 9-20, 1999
Zou H, Henzel WJ, Liu X, Lutschg A, Wang X: Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c dependent activation of caspase 3. Cell 90: 405-413, 1997
Petronilli V, Constantini P, Scorrano L, Colonna R, Passamonti S, Bernardi P: The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J Biol Chem 269: 16638-16642, 1994
Marchetti P, Decaudin D, Zamzami N, Hirsch T, Susin SA, Kroemer G: Redox regulation of apoptosis: Impact of thiol oxidation status on mitochondrial function. Eur J Immunol 27: 289-296, 1997
Li P, Deepak N, Budihardjo I, Srinivasalu SM, Ahmad M, Alnemri ES, Wang X: Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479-489, 1997
Wolf BB, Green DR: Suicidal tendencies: Apoptotic cell death by caspase family proteinases. J Biol Chem 274: 20049-20052, 1999
Tan S, Sagara Y, Liu Y, Maher P, Schubert D: The regulation of reactive oxygen species production during programmed cell death. Cell Biol 141:1423-1432, 1998
Suzuki S, Higuchi M, Proske RJ, Oridate N, Hong WK, Lotan R: Implication of mitochondria-derived reactive oxygen species, cytochrome C and caspase-3 in N-(4-hydroxyphenyl) retinamide-induced apoptosis in cervical carcinoma cells. Oncogene 18: 6380-6387, 1999
Li PF, Dietz R, von Harsdorf R: p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c independent apoptosis blocked by Bcl-2. EMBO J 18: 6027-6036, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huat, B.T.K., Swamy, S.M.K. Intracellular glutathione depletion and reactive oxygen species generation are important in α-hederin-induced apoptosis of P388 cells. Mol Cell Biochem 245, 127–139 (2003). https://doi.org/10.1023/A:1022807207948
Issue Date:
DOI: https://doi.org/10.1023/A:1022807207948