Abstract
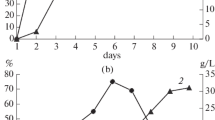
In order to identify qualitative markers of a potential mycoherbicide against Canada thistle based on the mycelium of the fungus Stagonospora cirsii VIZR 1.41, changes in its pathogenicity and lipid profiles during submerged cultivation on liquid sucrose-soybean medium were analyzed. The fatty-acid composition of major lipids and the identification of molecular species of structural lipids were determined. The lipid composition during fungal growth changed together with its pathogenicity. The highly pathogenic mycelium of the fungus (at the log phase of growth) was characterized with the relatively highest content of structural lipids: phosphatidylcholine (PC), phosphatidylethanolamine (PE), and ergosterol (ES). Their relative concentration decreased with culture age and a decrease in pathogenicity. The highest lipid yield was found at the beginning of stationary growth phase (at the maximal biomass yield) of S. cirsii; triacylglycerides (TAGs) were the most abundant. The double-bond index of fatty acids in glycerolipids decreased with culture age, and the substitution of molecular PC species (34:2 versus 36:4) and PE (36:4 versus 34:2) was observed as well. High levels of ES, TAG, PC (in particular, 34:2 forms) and PE (36:4 forms) were presumed to be qualitative markers of S. cirsii mycelium.




Similar content being viewed by others
REFERENCES
Rella, A., Farnoud, A.M., and Del Poeta, M., Prog. Lipid Res., 2016, vol. 61, pp. 63–67.
Kobae, Y., Gutjahr, C., Paszkowski, U., Kojima, T., Fujiwara, T., and Hata, S., Plant Cell Physiol., 2014, vol. 55, no. 11, pp. 1945–1953.
Aguilar, L.R., Pardo, J.P., Lomelí, M.M., Bocardo, O.I.L., Juárez Oropeza, M.A., and Guerra Sánchez, G., Arch. Microbiol., 2017, vol. 199, pp. 1195–1209.
Keyhani, N.O., Fungal Biol., 2018, vol. 122, no. 6, pp. 420–429.
Gao, Q., Shang, Y., Huang, W., and Wang, C., Appl. Environ. Microbiol., 2013, vol. 79, no. 24, pp. 7646–7653.
Harayama, T. and Riezman, H., Nat. Rev. Mol. Cell Biol., 2018, vol. 19, no. 5, pp. 281–296.
Siebers, M., Brands, M., Wewer, V., Duan, Y., Hölzl, G., and Dörmann, P., Biochim. Biophys. Acta, 2016, vol. 1861, no. 9, p. 1379.
Chen, Y.-L., Montedonico, A.E., Kauffman, S., Dunlap, J.R., Menn, F.-M., and Reynolds, T.B., Mol. Microbiol., 2010, vol. 75, no. 5, pp. 1112–1132.
Gao, Q., Yuzhen, L., Hongyan, Y., Xu, Y.-J., Huang, W., and Wang, C., Environ. Microbiol., 2016, vol. 18, no. 11, pp. 3976–3990.
Wang, J., Wang, H., Zhang, C., Wu, T., Ma, Z., and Chen, Y., Phytopathol. Res., 2019, vol. 1, no. 16. https://doi.org/10.1186/s42483-019-0023-9
Kazan, K. and Gardiner, D.M., PLoS Pathog, 2017, vol. 13, no. 5. https://doi.org/10.1371/journal.ppat.1006297
Warnecke, D. and Heinz, E., Cell. Mol. Life Sci., 2003, vol. 60, no. 5, pp. 919–941.
Ramamoorthy, V., Cahoon, E.B., Thokala, M., Kaur, J., Li, J., and Shah, D.M., Eukaryotic Cell, 2009, vol. 8, no. 2, pp. 217–229.
Weete, J.D., Lipid Biochemistry of Fungi and Other Organisms, New York: Plenum, 1980.
Beccaccioli, M., Reverberi, M., and Scala, V., Front. Biosci., 2019, vol. 24, no. 1, pp. 172–175.
Frolova, G.M., Sokornova, S.V., and Berestetskii, A.O., Appl. Biochem. Microbiol., 2019, vol. 55, no. 5, pp. 556–562.
Sokornova, S.V. and Berestetskii, A.O., S.-Kh. Biol., 2018, vol. 53, no. 5, pp. 1054–1061.
Barykina, R.P., Veselova, T.D., Devyatov, A.G., Dzhalilova, Kh.Kh., Il’ina, G.M., and Chubatova, N.V., Spravochnik po botanicheskoi mikrotekhnike (A Handbook on Botanical Microequipment), Moscow: Mosk. Univ., 2004.
Kotlova, E.R., Senik, S.V., Kyukher, T., Shavarda, A.L., Kiyashko, A.A., Psurtseva, N.V., and Zubarev, R.A., Microbiology (Moscow), 2009, vol. 78, no. 2, pp. 193–201.
Fuchs, B., Suss, R., Teuber, K., Eibisch, M., and Schiller, J., J. Chromatogr., A, 2011, vol. 1218, no. 19, pp. 2754–2774.
Benning, C., Huang, Z.H., and Gage, D.A., Arch. Biochem. Biophys., 1995, vol. 317, no. 1, pp. 103–111.
Berestetskiy, A. and Sokornova, S., in Biological Approaches for Controlling Weeds, Radhakrishnan, R., Ed., London: IntechOpen, 2018. https://doi.org/10.5772/intechopen.76936
Pavlova, N.A. and Sokornova, S.V., Vestn. Zashch. Rast., 2018, vol. 4, no. 98, pp. 67‒69.
Skoneczny, M. and Skoneczna, A., Stress Response Mechanisms in Fungi, Skoneczny, M., Ed., Cham: Springer, 2018, pp. 35–85.
Akpinar-Bayizit, A., Int. J. Chem. Eng. Appl., 2014, vol. 5, no. 5, pp. 409–414.
Solomon, P.S., Lee, R.C., Wilson, T.J., and Oliver, R.P., Mol. Microbiol., 2004, vol. 53, no. 4, pp. 1065–1073.
Thines, E., Weber, R.W.S., and Talbot, N.J., Plant Cell, 2000, vol. 12, no. 9, pp. 1703–1718.
Rudd, J.J., Kanyuka, K., Hassani-Pak, K., Derbyshire, M., Andongabo, A., Devonshire, J., et al., Plant Physiol., 2015, vol. 167, no. 3, pp. 1158–1185.
Lösel, D.M., Pestic. Sci., 1991, vol. 32, no. 3, pp. 353–362.
Van Etten, J. and Gottlieb, D., J. Bacteriol., 1965, vol. 89, no. 2, pp. 409–414.
Klemptner, R.L., Sherwood, J.S., Tugizimana, F., Dubery, I.A., and Piater, L.A., Mol. Plant Pathol., 2014, vol. 15, no. 7, pp. 747–761.
Govrin, E.M. and Levine, A., Curr. Biol., 2000, vol. 10, no. 13, pp. 751–757.
Ding, W., Palaiokostas, M., Wang, W., and Orsi, M., J. Phys. Chem., 2015, vol. 119, no. 49, pp. 15263–15274.
Janssen, M.J., Koorengevel, M.C., de Kruijff, B., and de Kroon, A.I., Yeast, 2000, vol. 16, no. 7, pp. 641–650.
Cassilly, C.D. and Reynolds, T.B., J. Fungi, 2018, vol. 4, no. 28. https://doi.org/10.3390/jof4010028
Nimrichter, L., Cerqueira, M.D., Leităo, E.A., Miranda, K., Nakayasu, E.S., Almeida, S.R., Almeida, I.C., Alviano, C.S., Barreto-Bergter, E., and Rodriges, M.L., Infect. Immun., 2005, vol. 73, no. 12, pp. 7860–7868.
Oura, T. and Kajiwara, S., Arch. Microbiol., 2008, vol. 154, no. 12, pp. 3795–3803.
Oura, T. and Kajiwara, S., Arch. Microbiol., 2010, vol. 156, no. 4, pp. 1234–1243.
ACKNOWLEDGMENTS
The authors are grateful to M. Z. Muradymov (Botanical Institute), M. A. Vinogradskaya (Botanical Institute), and A. V. Rubizhan (VIZR) for their substantial research assistance.
Funding
This work was carried out with the financial support of the Russian Science Foundation (project No. 16-16-00085) on the equipment of the Innovative Plant Protection Technologies Center for Collective Use (VIZR), as well as the research centers Development of Molecular and Cellular Technologies and Chemical Analysis and Materials of St. Petersburg State University.
The methodological basis of the protocol for the analysis of molecular species of glycoceramides and sterols was developed during the implementation of the topic “Assessment of changes in the correlation structure of metabolic networks during the growth and development of fungi and plants from the standpoint of system biology” of the Botanical Institute of the Russian Academy of Sciences (AAAA-A18-118032390136-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. The study was performed without the use of animals or people as subjects.
Additional information
Translated by P. Kuchina
Rights and permissions
About this article
Cite this article
Frolova, G.M., Kotlova, E.R., Sokornova, S.V. et al. Pathogenicity and Lipid Composition of Mycelium of the Fungus Stagonospora cirsii VIZR 1.41 during Submerged Cultivation. Appl Biochem Microbiol 57, 226–235 (2021). https://doi.org/10.1134/S0003683821020034
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683821020034