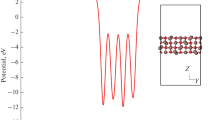
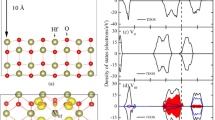
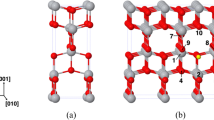
Abstract—
Density-functional calculations are used to study the structural and electronic properties of stoichiometric and imperfect In2O3 (011) surfaces. We calculate energies of formation of neutral oxygen vacancies on the surface of an indium oxide nanocrystal and analyze the adsorption of an oxygen atom in its ground (triplet) state on a model imperfect surface having O4 vacancies in different charge states. The results indicate that adsorption on a \(V_{{\text{O}}}^{{2 + }}\) vacancy is the most energetically favorable and that the oxygen atom involved switches from a triplet state to a singlet one. We consider oxygen molecule adsorption from different initial geometric configurations on neutral O1–6 vacancies.







Similar content being viewed by others
REFERENCES
Chen, P., Yin, X., Que, M., Liu, X., and Que, W., Low temperature solution processed indium oxide thin films with reliable photoelectrochemical stability for efficient and stable planar perovskite solar cells, J. Mater. Chem. A, 2017, vol. 5, pp. 9641–9648.
Baratto, C., Ferroni, M., Faglia, G., and Sberveglieri, G., Iron-doped indium oxide by modified RGTO deposition for ozone sensing, Sens. Actuators, B, 2006, vol. 118, nos. 1–2, pp. 221–225.
Yamaura, H., Jinkawa, T., Tamaki, J., Moriya, K., Miura, N., and Yamazoe, N., Indium oxide based gas sensor for selective detection of CO, Sens. Actuators, B, 1996, vol. 36, nos. 1–3, pp. 325–332.
Golan, G., Axelevitch, A., Gorenstein, B., and Peled, A., Novel type of indium oxide thin films sputtering for opto-electronic applications, Appl. Surf. Sci., 2007, vol. 253, no. 15, pp. 6608–6611.
Fang, Z., Assaaoudi, H., Guthrie, R.I.L., Kozinski, J.A., and Butler, I.S., Continuous synthesis of tin and indium oxide nanoparticles in sub- and supercritical water, J. Am. Ceram. Soc., 2007, vol. 90, no. 8, pp. 2367–2371.
Marezio, M., Refinement of the crystal structure of In2O3 at two wavelengths, Acta Crystallogr., 1966, vol. 20, no. 6, pp. 723–728.
Hao, Y., Meng, G., Ye, C., and Zhang, L., Controlled synthesis of In2O3 octahedrons and nanowires, Cryst. Growth Des., 2005, vol. 5, no. 4, pp. 1617–1621.
Shi, M., Xu, F., Yu, K., Zhu, Z., and Fang, J., Controllable synthesis of In2O3 nanocubes, truncated nanocubes, and symmetric multipods, J. Phys. Chem. C, 2007, vol. 111, no. 44, pp. 16267–16271.
Agoston, P. and Albe, K., Thermodynamic stability, stoichiometry, and electronic structure of bcc-In2O3 surfaces, Phys. Rev. B:, Condens. Matter Mater. Phys., 2011, vol. 84, paper 045311.
Walsh, A. and Catlow, C.R.A., Structure, stability and work functions of the low index surfaces of pure indium oxide and Sn-doped indium oxide (ITO) from density functional theory, J. Mater. Chem., 2010, vol. 20, no. 46, pp. 10438–10444.
Zhou, C., Li, J., Chen, S., Wu, J., Heier, K.R., and Cheng, H., First-principles study on water and oxygen adsorption on surfaces of indium oxide and indium tin oxide nanoparticles, J. Phys. Chem. C, 2008, vol. 112, no. 36, pp. 14015–14020.
Inerbaev, T., Sahara, R., Mizuseki, H., Kawazoe, Y., and Nakamura, T., Theoretical modeling of oxygen and water adsorption on indium oxide (111) surface, ACS Symp. Ser., 2015, vol. 1196, pp. 137–149.
Tasker, P.W., The stability of ionic crystal surfaces, J. Phys. C: Solid State Phys., 1979, vol. 12, no. 22, pp. 4977–4984.
Giannozzi, P., Baroni, S., Bonini, N., Calandra, M., Car, R., Cavazzoni, C., Ceresoli, D., Chiarotti, G.L., Cococcioni, M., Dabo, I., Dal Corso, A., Fabris, S., Fratesi, G., de Gironcoli, S., Gebauer, R., Gerstmann, U., Gougoussis, C., Kokalj, A., Lazzeri, M., Martin-Samos, L., Marzari, N., Mauri, F., Mazzarello, R., Paolini, S., Pasquarello, A., Paulatto, L., Sbraccia, C., Scandolo, S., Sclauzero, G., Seitsonen, A.P., Smogunov, A., Umari, P., and Wentzcovitch, R.M., A modular and open-source software project for quantum simulations of materials, J. Phys.: Condens. Matter, 2009, vol. 21, paper 395502. http://arxiv.org/abs/0906.2569
Kresse, G. and Joubert, D., From ultrasoft pseudopotentials to the projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, vol. 59, pp. 1758–1775.
Perdew, P., Burke, K., and Ernzerhof, M., Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, vol. 77, pp. 3865–3868.
Monkhorst, H.J. and Pack, J.D., Special points for Brillouin-zone integrations, Phys. Rev. B: Solid State, 1976, vol. 13, pp. 5188–5192.
Smith, J.F. and Schneider, V.L., Anisotropic thermal expansion of indium, J. Less-Common Met., 1964, vol. 7, no. 1, pp. 17–22.
Cordfunke, E.H.P., Konings, R.J.M., and Ouweltjes, W., The standard enthalpy of formation of In2O3, J. Chem. Thermodyn., 1991, vol. 23, no. 5, pp. 451–454.
Huber, K.P. and Herzberg, G., Molecular Spectra and Molecular Structure, New York: Van Nostrand, 1979, vol. 4.
Löwdin, P.O., On the non-orthogonality problem, Adv. Quantum Chem., 1970, vol. 5, pp. 185–199.
Che, M. and Tench, A.J., Characterization and reactivity of molecular-oxygen species on oxide surfaces, Adv. Catal., 1983, vol. 32, pp. 1–148.
Bourlange, A., Payne, D.J., Egdell, R.G., Foord, J.S., Edwards, P.P., Jones, M.O., Schertel, A., Dobson, P.J., and Hutchison, J.L., Growth of In2O3(100) on Y-stabilized ZrO2(100) by O-plasma assisted molecular beam epitaxy, Appl. Phys. Lett., 2008, vol. 92, no. 9, pp. 092117–092120.
ACKNOWLEDGMENTS
We are grateful to our colleagues at the Joint Supercomputer Center, Russian Academy of Sciences, for providing us with necessary computational resources.
Funding
This work was supported by the Russian Federation Ministry of Science and Higher Education (state research target no. 0082-2018-0003 for the Semenov Federal Research Center for Chemical Physics, Russian Academy of Sciences, theme no. 45.22: Fundamental Principles behind the Development of a New Generation of Nanostructured Systems with Unique Performance Parameters, state registration no. AAAA-A18-118012390045-2) and the Russian Foundation for Basic Research (grant nos. 19-37-90016 and 19-07-00141a).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Kurmangaleev, K.S., Mikhailova, T.Y. & Trakhtenberg, L.I. Oxygen Chemisorption on the Surface of an In2O3 (011) Nanocrystal. Inorg Mater 56, 1138–1146 (2020). https://doi.org/10.1134/S0020168520110060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168520110060