Abstract
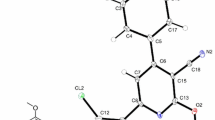
In this work, 6-tert-butyl 3-ethyl 2-amino-4,5-dihydrothieno[2,3-c]pyridine-3,6(7H)-dicarboxylate is synthesized from starting tert-butyl 4-oxopiperidine-1-carboxylate, ethyl cyanomalonate, and sulfur, and then, coupled with same aromatic aldehyde affords the corresponding Schiff base compounds. These compounds (2a–d) are characterized using FTIR, 1H and 13C NMR spectroscopic methods. The crystal and molecular structure of (E)-6-tert-butyl 3-ethyl 2-((2-hydroxy-3-methoxybenzylidene)amino)-4,5-dihydrothieno[2,3-c]pyridine-3,6(7H)-dicarboxylate (2a) is characterized by the X-ray crystallographic analysis. Compound 2a crystallizes in the monoclinic space group P21/c. The molecular and crystal structure is stabilized by two O–H⋯N and O–H⋯O intramolecular hydrogen bonds (O⋯N and O⋯O are 2.598(5) Å and 2.990(5) Å, respectively; O–H⋯N = 147° and O–H⋯O = 134°). According to DFT, compound 2d also shows the intramolecular hydrogen bonding, while there is no this type of interaction in compound 2b and 2c.






Similar content being viewed by others
REFERENCES
R. W. Sabnis, D. W. Rangnekar, and N. D. Sonawane. J. Heterocycl. Chem., 1999, 36, 333.
G. G. Mohamed, M. M. Omar, and A. M. M. Hindy. Spectrochim. Acta, Part A: Mol. Biomol. Spectrosc., 2005, 62, 1140.
S. Kathiresan, S. Mugesh, J. Annaraj, and M. Murugan. New J. Chem., 2017, 41, 1267.
A. S. Abu-Surrah, M. Kettunen, M. Leskela, and Y. Z. Al-Abed. Anorg. Allg. Chem., 2008, 634, 2655.
D. R. Allen, A. Bolt, G. A. Chapman, R. L. Knight, J. W. G. Meissner, D. A. Owen, and R. J. Watson. Bioorg. Med. Chem. Lett., 2007, 17, 697.
X. Hu and T. J. Maimone. J. Am. Chem. Soc., 2014, 136, 5287.
L. G. Kapitsa, E. V. Suslov, G. V. Teplov, D. V. Korchagina, N. L. Komarova, K. P. Volcho, T. A. Voronina, A. I. Shevela, and N. F. Salakhutdinov. J. Pharm. Chem., 2012, 46, 263.
K. Singh, M. S. Barwa, and P. Tyagi. Eur. J. Med. Chem., 2006, 41, 147.
S. N. Pandeya, D. Sriram, G. Nath, and E. D. Clercq. Eur. J. Pharm. Sci., 1999, 9, 25.
P. K. Panchal, H. M. Parekh, and P. B. Pansuriya. J. Enzyme Inhib. Med. Chem., 2006, 21, 203.
S. M. Borisov, R. Saf, R. Fischer, and I. Klimant. Inorg. Chem., 2013, 52, 1206.
J. Chai, Y. Wu, B. Yang, and B. Liu. J. Mater. Chem., C, 2018, 6, 4057.
M. Yan, T. Li, and Z. Yang. Inorg. Chem. Commun., 2011, 3, 463.
X. Tang, J. Han, Y. Wang, L. Ni, X. Bao, L. Wang, and W. Zhang. Spectrochim. Acta, Part. A: Mol. Biomol. Spectrosc., 2017, 173, 721.
C. Pan, K. Wang, S. Ji, H. Wang, Z. Li, H. He, and Y. Huo. RSC Adv., 2017, 7, 36007.
V. Nishal, D. Singh, A. Kumar, V. Tanwar, I. Singh, R. Srivastava, and P. S. Kadyan. J. Org. Semicond., 2014, 2, 15.
J. H. Jia, X. M. Tao, Y. J. Li, and W. J. Sheng. Chem. Phys. Lett., 2011, 524, 114.
W. A. Zoubi and Y. G. Ko. Appl. Organomet. Chem., 2017, 31, 3574.
A. W. Jeevadason, K. K. Murugavel, and M. A. Neelakantan. Renewable Sustainable Energy Rev., 2014, 36, 220.
J. Zhang, L. Xu, and W. Y. Wong. Coord. Chem. Rev., 2018, 355, 180.
M. Sedighipoor, A. H. Kianfar, G. Mohammadnezhad, H. Gorls, and W. Plass. Inorg. Chim. Acta, 2018, 476, 20.
N. Raman, R. Jeyamurugan, R. Senthilkumar, B. Rajkapoor, and S. G. Franzblau. Eur. J. Med. Chem., 2010, 45, 5438.
M. Mesbah, T. Douadi, F. Sahli, S. Issaadi, S. Boukazoula, and S. Chafaa. J. Mol. Struct., 2018, 1151, 41.
M. Mohamadi, S. Y. Ebrahimipour, M. Torkzadeh-Mahani, S. Foro, and A. Akbari. RSC Adv., 2015, 5, 101063.
M. Z. Ghdhayeb, R. A. Haque, S. Budagumpi, M. B. Khadeer Ahamed, and A. M. S. A. Majid. Inorg. Chem. Commun., 2017, 75, 41.
N. P. Peet, S. Sunder, R. J. Barbuch, and A. P. Vinogradoff. J. Heterocycl. Chem., 1986, 23, 129–134.
APEX2, SAINT and SADABS. Bruker AXS: Madison, Wisconsin, USA, 2012.
G. M. Sheldrick. SHELXS97. Univ. of Göttingen: Germany:, 1997.
G. M. Sheldrick. SHELXL97. Univ. of Göttingen: Germany:, 1997.
C. K. Johnson. ORTEP, Report ORNL-3794. Oak Ridge National Laboratory: Tenessee, USA, 1965.
M. Nardelli. J Appl. Crystallogr., 1995, 28, 659.
A. L Spek. PLATON. University of Utrecht: The Netherlands, 2003.
P. Walters, M. Stahl. Babel, Version 1.1. Tucson, AZ: Department of Chemistry, University of Arizona, 1994.
D. Roy, K. Todd, M. John. GaussView, Version 5. Shawnee Mission, KS: Semichem, 2009.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox. Gaussian 09, Revision C.01. Gaussian: Wallingford, CT, 2010.
Funding
The authors acknowledge the Aksaray University Science and Technology Application and Research Center, Aksaray, Turkey, for the use of the Bruker SMART BREEZE CCD diffractometer (purchased under grant No. 2010K120480 of the State of Planning Organization). The numerical calculations reported in this paper were fully performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Çolak, N., Karayel, A., Buldurun, K. et al. SYNTHESIS, CHARACTERIZATION, THERMAL, X-RAY, AND DFT ANALYSES OF 6-TERT-BUTYL 3-ETHYL 2-[(3-METHOXY/5-BROMO)-2-HYDROXY AND (3-NITRO/3-METHOXY)BENZYLIDENEAMINO]-4,5-DIHYDROTHIENO[2,3-C]PYRIDINE-3,6(7H)-DICARBOXYLATE. J Struct Chem 62, 37–46 (2021). https://doi.org/10.1134/S0022476621010054
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621010054