Abstract
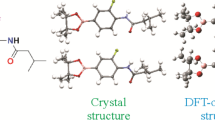
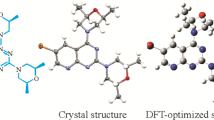
1-(2-Bromobenzyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole is an organic intermediate with both pyrazole heterocycle and borate functional group. In this paper, the title compound is obtained by the nucleophilic substitution reaction. The structure of the compound is confirmed by FTIR, 1H and 13C NMR spectroscopy, and MS. At the same time, the single crystal of the title compound is measured by X-ray diffraction and is subjected to the crystallographic and conformational analysis. The molecular structure is further calculated using Density Functional Theory (DFT) and compared with the X-ray diffraction value. The results of the conformational analysis indicate that the molecular structure optimized by DFT is consistent with the crystal structure determined by single crystal X-ray diffraction. In addition, the molecular electrostatic potential and frontier molecular orbitals of the title compound are further investigated by DFT, revealing molecular structure characteristics and molecular conformations.







Similar content being viewed by others
REFERENCES
R. Sreedevi, S. Saranya, K. R. Rohit, and G. Anilkumar. Adv. Synth. Catal., 2019, 361(10), 2236-2249.
J. F. Campos, M. Loubidi, M. C. Scherrmann, and S. Berteina-Raboin. Molecules, 2018, 23(3), 684.
K. Khalid, R. Smaail, R. Youssef, T. Jamal, M. Yahia, and A. A Faiz, A M. Hammed. Molecules, 2018, 23, 134.
S. Fustero, M. Sanchez-Rosello, P. Barrio, and A. Simónfuentes. Chem. Rev., 2011, 111(11), 6984-7034.
A. Ansari, A. Ali, and M. Asif. New J. Chem., 2017, 41(1), 16-41.
M. J. Masuda-Herrera, K. L. Dobo, M. O. Kenyon, J. D. Kenny, and J. P. Bercu. Environ. Mol. Mutagen., 2019, 60(9), 766-777.
N. J. Hiller, N. A. do Amaral e Silva, T. A. Tavares, R. X. Faria, M. N. Eberlin, and D. de Luna Martins. Eur. J. Org. Chem., 2020, 4841-4877.
J. S. Zhao, P. Jin, N. Xi, and D. D. Wei. Chin. J. Struct. Chem., 2017, 36, 937-942.
W. Y. Lin, F. Yang, A. N. Duan, W. W. You, and P. L. Zhao. Chin. J. Struct. Chem., 2018, 37, 1557-1562.
G. M. Sheldrick. SHELXS-2018/3. Program for Solution of Crystal Structures. University of Göttingen: Göttingen, Germany, 2018.
G. M. Sheldrick. SHELXL-2018/3. Program for Refinement of Crystal Structures. University of Göttingen: Göttingen, Germany, 2018.
M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, and G. Petersson. Gaussian 09, Revision C.01. Gaussian: Wallingford, 2010.
A. Frish, A. B. Nielsen, and A. J. Holder. Gauss View User Manual. Gaussian: Pittsburg, PA, 2011.
Y. Y. Liu, K. Z. Lv, Y. Li, Q. L. Nan, and J. Y. Xu. Chin. J. Struct. Chem., 2019, 38, 171-186.
B. J. Deppmeier, A. J. Driessen, T. S. Hehre, W. J. Hehre, J. A. Johnson, P. E. Klunzinger, J. M. Leonard, I. N. Pham, W. J. Pietro, and J. Yu. Spartan08. Wavefunction: Irvine, CA, 2009.
H. H. Brintzinger, M. H. Prosenc, F. Schaper, A. Weeber, and U. Wieser. J. Mol. Struct., 1999, 485-486, 409-419.
N. Huang, C. Kalyanaraman, K. Bernacki, and M. P. Jacobson. Phys. Chem. Chem. Phys., 2006, 8, 5166-5177.
K. Fukui, T. Yonezawa, and H. Shingu. J. Chem. Phys., 2004, 20(10), 1653.
M. D. Rozeboom, I. M. Tegmo-Larsson, and K. Houk. J. Org. Chem., 1981, 46(11), 2338-2345.
C.-G. Zhan, J. A. Nichols, and D. A. Dixon. J. Phys. Chem. A, 2003, 107(20), 4184-4195.
R. G. Parr and R. G. Pearson. J. Am. Chem. Soc., 1984, 15(13), 7512-7516.
R. G. Pearson. Proc. Natl. Acad. Sci., 1986, 83(22), 8440-8441.
R. G. Parr and P. K. Chattaraj. J. Am. Chem. Soc., 1991, 113(5), 1854-1855.
Funding
This work was supported by Guizhou University of Traditional Chinese Medicine 2018 annual academic new seedling cultivation and innovation exploration special project cultivation project plan. (Qiankehe platform talent [2018]5766-14).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 6, pp. 997-1006.https://doi.org/10.26902/JSC_id72898
Supplementary material
Rights and permissions
About this article
Cite this article
Yang, Z., Huang, P., Chen, J. et al. SYNTHESIS, CRYSTAL STRUCTURE, AND DFT STUDY OF 1-(2-BROMOBENZYL)-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-1H-PYRAZOLE. J Struct Chem 62, 928–937 (2021). https://doi.org/10.1134/S0022476621060123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621060123