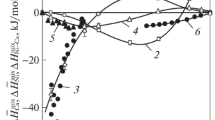
Abstract
The partial and integral thermodynamic functions of mixing and vaporization are calculated for Zn–Са alloys from the vapor pressures determined by the boiling point method. Alloy formation over the whole range of concentrations proceeds with heat release, with attendant considerable ordering in the system relative to an ideal solution up to ~70 at % Са in the alloy and with a minor increase in disorder when calcium concentration exceeds this value. The curves of integral functions of vaporization feature extremes at ~85 at % Ca: a minimum entropy of 85.48 J/(mol K) and a maximum enthalpy of 156.44 kJ/mol. The vapor pressures of alloy components were used to supplement the Zn–Са phase diagram with the liquid and vapor coexistence fields at the atmospheric pressure (101.33 kPa) and in a vacuum (1.33 and 0.7 kPa). The existence of a liquid containing 93.2 ± 6.7 at % Ca that boils unseparably at 1494°С was discovered. As the pressure (boiling temperature) decreases, the azeotrope composition shifts (in accordance with the Vrevsky law) toward the zinc edge of the phase diagram, where zinc has a lower enthalpy of vaporization compared to that of calcium (118.6 kJ/mol against 153.7 kJ/mol). The positions of boundaries of the vapor–liquid equilibrium fields indicate the impracticability of distillation separation of the Zn–Са system to the constituent metals.





Similar content being viewed by others
REFERENCES
G. E. R. Schulze and U. Werncke, Monats. Deutsch. Akad. Wissensch. 5, 68 (1963).
P. Chiotti and R. J. Hecht, Trans. Met. Soc. 239, 536 (1967).
B. P. Burylev, Thermodynamic and Thermochemical Constants (Nauka, Moscow, 1970) [in Russian].
A. E. Vol and I. K. Kagan, Structure and Properties of Binary Inorganic Systems (Nauka, Moscow, 1979), Vol. 4 [in Russian].
Phase Diagrams of Binary Metal Systems, Ed. by N. P. Lyakishev (Mashinostroenie, Moscow, 1996), Vol. 1 [in Russian].
R. Hultgren, P. D. Desai, and D. T. Hawkins, et al. Selected Values of the Thermodynamic Properties of Binary Alloys (ASM, New York, 1973).
V. I. Zhuravlev, A. V. Volkovich, and G. N. Zhirkov, Proceedings of the 7th Workshop on the Physical and Electrochemistry of Rare and Nonferrous Metals (Apa-tity, 1992), p. 38.
Y.-N. Dai and B. Yang, Vacuum Metallurgy of Non-Ferrous Metals (Metallurgical Ind. Press, Beijing, 2000), Vol. 3.
C. O. Brubaker and Z.-K. Liu, Calphad 25, 381 (2001). https://doi.org/10.1016/S0364-5916(01)00057-8
Y. Zhong, K. Ozturk, and Z.-K. Liu, J. Phase. Eq. 24, 340 (2003).
P. J. Spencer, A. D. Pelton, Y.-B. Kang, et al., Calphad 32, 423 (2008). https://doi.org/j.calphad.200803.01
M. V. Shtenberg, V. A. Bychinskii, O. I. Koroleva, et al., Zh. Neorg. Khim. 62, 1470 (2017). https://doi.org/10.7868/S0044457X17110071
I. Yu. Shilov and A. K. Lyashchenko, Zh. Neorg. Khim. 64, 1006 (2019). https://doi.org/10.1134/S0044457X19090216
V. G. Muradov, Uch. Zap. Ul’yanovsk. Gos. Pedag. Inst. 18 (5), 64 (1964).
E. Schürmann and R. Schmid, Arch. Eisenhuttenwes 46, 773 (1975).
V. M. Glazov, V. B. Lazarev, and V. V. Zharov, Phase Diagrams of Simple Substances (Nauka, Moscow, 1980) [in Russian].
V. P. Malyshev, A. M. Turdukozhaeva, E. A. Ospanov, and B. Sarkenov, Evaporation and Boiling of Simple Substances (Nauchnyi Mir, Moscow, 2010) [in Russian].
V. N. Volodin, V. E. Khrapunov, B. K. Kenzhaliev, et al., Izv. Vyssh. Uchebn. Zaved., Tsvetn. Metal., No. 3, 22 (2005).
V. N. Volodin and Yu. Zh. Tuleushev, Zh. Fiz. Khim. 94, 975 (2020)
V. N. Volodin, Yu. Zh. Tuleushev, N. M. Burabaeva, and A. S. Kerimshe, Zh. Neorg. Khim. 65, 2020. https://doi.org/10.31857/S0044457X20050256
Y. K. Rao, Metall. Trans. A 14, 308 (1983).
A. G. Morachevskii, Thermodynamics of Molten Metal and Salt Systems (Metallurgiya, Moscow, 1987) [in Russian].
J. B. Clark and P. W. Richter, Proceedings of the 7th International AIRAPT Conference, Le Creusot, 1979 (Oxford, 1980), Vol. 1, p. 363.
V. N. Volodin, V. E. Khrapunov, and I. A. Marki, Zh. Fiz. Khim. 85, 1392 (2011).
M. S. Vrevsky, Works on the Theory of Solutions (Izd–vo Akad. Nauk SSSR, Moscow/Leningrad, 1953) [in Russian].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Fedorova
Rights and permissions
About this article
Cite this article
Volodin, V.N., Tuleushev, Y.Z., Burabaeva, N.M. et al. Thermodynamics of Solutions and Azeotropy in Zinc–Calcium Melts. Russ. J. Inorg. Chem. 65, 1069–1076 (2020). https://doi.org/10.1134/S0036023620070232
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620070232