Abstract
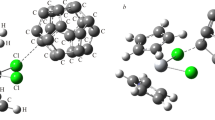
In this study, we have investigated the interaction between Al12N12 nano-cluster and titanocene dichloride anticancer drug complex using B3P86 functional. Two interactions modes between Al12N12 nano-cluster and titanocene dichloride complex (TiCl2Cp2, Cp = η5-(C5H5)2) have been studied. The bonding interaction between the Al12N12 nano-cluster and anticancer drug has been examined through energy decomposition analysis (EDA). In addition, Shubin Liu’s energy decomposition analysis (EDA-SBL) has been used to study the source of energy different between various isomers of Al12N12···cp2TiCl2 complex. Charge transfer between fragments have been illustrated with electrophilicity-based charge transfer (ECT). The related molecular properties to the biological activity of these drug precursor molecules have been studied (octanol–water partition coefficient (log P), molecular volume (Vm)). The quantum theory of atoms in molecules (QTAIM) analysis has been applied to assess the Al–Cl bonds within the Al12N12···cp2TiCl2 complexes.



Similar content being viewed by others
REFERENCES
P. Koepf-Maier and H. Koepf, Chem. Rev. 87, 1137 (1987).https://doi.org/10.1021/cr00081a012
P. Köpf-Maier and H. Köpf, Bioinorg. Chem. Struct. Bond. 70, 103 (1988).https://doi.org/10.1007/3-540-50130-4_3
M. J. Clarke, F. Zhu, and D. R. Frasca, Chem. Rev. 99, 2511 (1999).https://doi.org/10.1021/cr9804238
E. V. Tshuva and D. Peri, Coord. Chem. Rev. 253, 2098 (2009).https://doi.org/10.1016/j.ccr.2008.11.015
S. Gómez-Ruiz, G. N. Kaluderovíc, D. Polo-Cerón, et al., J. T. Sabo, Inorg. Chem. Commun. 10, 748 (2007).https://doi.org/10.1016/j.inoche.2007.03.016
S. Gómez-Ruiz, G. N. Kaluderovíc, D. Polo-Cerón, et al., J. Inorg. Biochem. 102, 1558 (2008).https://doi.org/10.1016/j.jinorgbio.2008.02.001
S. Barroso, A. M. Coelho, S. Gómez-Ruiz, et al., Dalton Trans. 43, 17 422 (2014).https://doi.org/10.1039/C4DT00975D
M. L. McLaughlin, J. M. Cronan, T. R. Schaller, and R. D. Snelling, J. Am. Chem. Soc. 112, 8949 (1990).https://doi.org/10.1021/ja00180a046
M. M. Harding, G. J. Harden, and L. D. Field, FEBS Lett. 322, 291 (1993).https://doi.org/10.1016/0014-5793(93)81588-q
J. H. Murray and M. M. Harding, J. Med. Chem. 37, 1936 (1994).https://doi.org/10.1021/jm00039a005
X. Chen and L. Zhou, Journal of Molecular Structure: THEOCHEM 940, 45 (2010).https://doi.org/10.1016/j.theochem.2009.10.007
O. R. Allen, L. Croll, A. L. Gott, et al., Organometals 23, 288 (2004).https://doi.org/10.1021/om030403i
J. R. Boyles, M. C. Baird, B. G. Campling, N. Jain, J. Inorg. Biochem. 84, 159 (2011).https://doi.org/10.1016/S0162-0134(00)00203-8
P. W. Causey and M. C. Baird, Organometals 4486 (2004).https://doi.org/10.1021/om049679w
R. Teuber and G. L. G. M. Tacke, J. Organomet. Chem. 545–546, 105 (1997).https://doi.org/10.1016/S0022-328X(97)00267-2
S. Fox, J. P. Dunne, D. Dronskowski, et al., Eur. J. Inorg. Chem. 3039 (2002).https://doi.org/10.1002/1099-0682(200211)2002:11<30-39::AID-EJIC3039>3.0.CO;2-0
F.-J. K. Rehmann, L. P. Cuffe, O. Mendoza, et al., Appl. Organomet. Chem. 19, 293 (2005).https://doi.org/10.1002/aoc.864
K. M. Kane, P. J. Shapiro, V. A. R. Cubbon, and A. L. Rheingold, Organometals 16, 4567 (1997).https://doi.org/10.1021/om9704399
M. Tacke, L. T. Allen, L. Cuffe, et al., J. Organomet. Chem. 689, 2242 (2004).https://doi.org/10.1016/j.jorganchem.2004.04.015
D. L. Strout, J. Phys. Chem. A 104, 3364 (2000).https://doi.org/10.1021/jp994129a
R. Wang, D. Zhang, and C. Liu, Chem. Phys. Lett. 411, 333 (2005).https://doi.org/10.1016/j.cplett.2005.06.055
B. Bertolus, F. Finocchi, and P. Millie, J. Chem. Phys. 120, 4333 (2004).https://doi.org/10.1063/1.1636717
C.-C. Fu, M. Weissmann, M. Machado, and P. Ordejón, Phys. Rev. B 63, 85 411 (2001).https://doi.org/10.1103/PhysRevB.63.085411
M. Bilge, J. Struc. Chem. 59, 1271 (2018).https://doi.org/10.1134/S0022476618060045
A. K. Kandalam, M. A. Blanco, and R. Pandey, J. Phys. Chem. B 105, 6080 (2001).https://doi.org/10.1021/jp004404p
E. S. E. Tahmasebi and Z. Biglari, Appl. Surf. Sci. 363, 197 (2016).https://doi.org/10.1016/j.apsusc.2015.12.001
Q. W. F. Zhang, X. Wang, N. Liu, et al., J. Phys. Chem. C 113, 4053 (2009).https://doi.org/10.1021/jp811484r
H.-S. Wu, F.-Q. Zhang, X.-H. Xu, et al., J. Phys. Chem. A 107, 204 (2003).https://doi.org/10.1021/jp027300i
H. Wu, X. Fan, and J.-L. Kuo, Int. J. Hydrogen En. 37, 14 336 (2012).https://doi.org/10.1016/j.ijhydene.2012.07.081
H. Ghanbari, B. G. Cousins, and A. M. Seifalian, Macromol. Rapid Commun. 32, 1032 (2011).https://doi.org/10.1002/marc.201100126
Z. Kazemi, R. Ghiasi, and S. Jamehbozorgi, J. Struct. Chem. 59, (2018).https://doi.org/10.1134/S0022476618050050
A. S. Ghasemi, F. Ashrafi, S. A. Babanejad, and A. Elyasi, J. Struc. Chem. 60, 13 (2019).https://doi.org/10.1134/S0022476619010037
E. Borowiak-Palen, E. Mendoza, A. Bachmatiuk, et al., Chem. Phys. Lett. 421, 129 (2006).https://doi.org/10.1016/j.cplett.2006.01.072
A. N. Khlobystov, D. A. Britz, and G. A. D. Briggs, Acc. Chem. Res. 38, 901 (2005).https://doi.org/10.1021/ar040287v
K. Yanagi, Y. Miyata, and H. Kataura, Adv. Mater. 18, 437 (2006).https://doi.org/10.1002/adma.200501839
S. A. Houston, N. S. Venkataramanan, A. Suvitha, and N. J. Wheate, Austral. J. Chem. 69, 1124 (2016).https://doi.org/10.1071/CH16067
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al, Gaussian 09, Revision A.02 (Gaussian, Wallingford CT, 2009).
P. J. Hay, J. Chem. Phys. 66, 4377 (1977).https://doi.org/10.1063/1.433731
R. Krishnan, J. S. Binkley, R. Seeger, and J. A. Pople, J. Chem. Phys. 72, 650 (1980).https://doi.org/10.1063/1.438955
A. D. McLean and G. S. Chandler, J. Chem. Phys. 72, 5639 (1980).https://doi.org/10.1063/1.438980
A. J. H. Wachters, J. Chem. Phys. 52, 1033 (1970).https://doi.org/10.1063/1.1673095
A. D. Becke, J. Chem. Phys. 98, 5648 (1993).https://doi.org/10.1063/1.464913
J. P. Perdew, Phys. Rev. B 33, 8822 (1986).https://doi.org/10.1103/PhysRevB.33.8822
Y. Zhao and D. G. Truhla, J. Phys. Chem. A 110, 5121 (2006).
M. Head-Gordon, J. A. Pople, and M. J. Frisch, Chem. Phys. Lett. 153, 503 (1988).
S. Saebø and J. Almlöf, Chem. Phys. Lett. 154, 83 (1989).
M. J. Frisch, M. Head-Gordon, and J. A. Pople, Chem. Phys. Lett. 166, 275 (1990).
M. J. Frisch, M. Head-Gordon, and J. A. Pople, Chem. Phys. Lett. 166, 281 (1990).
M. Head-Gordon and T. Head-Gordon, Chem. Phys. Lett. 220, 122 (1994).
N. M. O’Boyle, A. L. Tenderholt, and K. M. Langer, J. Comput. Chem. 29, 839 (2008).https://doi.org/10.1002/jcc.20823
T. Lu, F. Chen, J. Comp. Chem. 33, 580 (2012).https://doi.org/10.1002/jcc.22885
S. Liu, J. Chem. Phys. 126, 244 103 (2007).https://doi.org/10.1063/1.2747247
T. Lu and F. Chen, J. Mol. Graph. Model. 38, 314 (2012).https://doi.org/10.1016/j.jmgm.2012.07.004
T. Kim and H. Park, J. Mol. Graph. Model. 60, 108 (2015).https://doi.org/10.1016/j.jmgm.2015.06.004
J. Tomasi, B. Mennucci, and R. Cammi, Chem. Rev. 105, 2999 (2005).https://doi.org/10.1021/cr9904009
P. W. Ayers and R. G. Parr, J. Am. Chem. Soc. 122, 2010 (2000).https://doi.org/10.1021/ja9924039
R. G. Parr and P. K. Chattaraj, J. Am. Chem. Soc. 113, 1854 (1991).https://doi.org/10.1021/ja00005a072
R. G. Pearson, J. Chem. Educ. 64, 561 (1987).https://doi.org/10.1021/ed064p561
R. G. Pearson, Acc. Chem. Res. 26, 250 (1993).https://doi.org/10.1021/ar00029a004
R. G. Pearson, J. Chem. Educ. 76, 267 (1999).https://doi.org/10.1021/ed076p267
J. Padmanabhan, R. Parthasarathi, V. Subramanian, and P. K. Chattaraj, J. Phys. Chem. A 111, 1358 (2007).https://doi.org/10.1021/jp0649549
R. G. Pearson, J. Org. Chem. 54, 1430 (1989).https://doi.org/10.1021/jo00267a035
R. G. Parr and R. G. Pearson, J. Am. Chem. Soc. 105, 7512 (1983).https://doi.org/10.1021/ja00364a005
P. Geerlings, F. D. Proft, and W. Langenaeker, Chem. Rev. 103, 1793 (2003).https://doi.org/10.1021/cr990029p
R. G. Parr, L. Szenpály, and S. Liu, J. Am. Chem. Soc. 121, 1922 (1999).https://doi.org/10.1021/ja983494x
R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules (Oxford Univ. Press, Oxford, New York, 1989).
L. Sobczyk, S. J. Grabowski, and T. M. Krygowski, Chem. Rev. 105, 3513 (2005).https://doi.org/10.1021/cr030083c
R. F. W. Bader, C. F. Matta, and F. Cortés-Guzman, Organometallics 23, 6253 (2004).https://doi.org/10.1021/om049450g
X. Fradera, M. A. Austen, and R. F. W. Bader, J. Phys. Chem. A 103, 304 (1999).https://doi.org/10.1021/jp983362q
R. F. W. Bader and D.-F. Fang, J. Chem. Theor. Comput. 1, 403 (2005).https://doi.org/10.1021/ct049839l
P. M. Mitrasinovic, Can. J. Chem. 81, 542 (2003).https://doi.org/10.1139/v03-043
D. Cremer and E. Kraka, Croat. Chem. Acta 57, 1259 (1984).
M. Palusiak, J. Organometallic. Chem. 692, 3866 (2005).https://doi.org/10.1016/j.jorganchem.2007.05.029
P. Macchi and A. Sironi, Coord. Chem. Rev. 239, 383 (2003).https://doi.org/10.1016/S0010-8545(02)00252-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mozhdeh Shabani, Ghiasi, R., Zare, K. et al. Quantum Chemical Study of Interaction between Titanocene Dichloride Anticancer Drug and Al12N12 Nano-Cluster. Russ. J. Inorg. Chem. 65, 1726–1734 (2020). https://doi.org/10.1134/S0036023620110169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620110169