Abstract
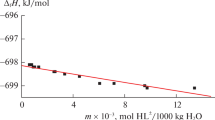
Heats of dissolution of crystalline L-glutathione in water and solutions of potassium hydroxide at 298.15 K are measured via direct calorimetry. Numerical values of the standard enthalpies of formation of glutathione in the crystalline state are calculated by additive groups method, based on group systematics with Benson-type classification of fragments that considers the effect of the primary environment on atoms. Standard enthalpies of formation of the tripeptide and the products of its dissociation in aqueous solutions are calculated.
Similar content being viewed by others
REFERENCES
C. Garcia-Ruiz and J. C. Fernández-Checa, J. Gastroenterol. Hepatol. 22, 38 (2007).
S. K. Biswas and I. Rahman, Mol. Asp. Med. 30, 60 (2009).
J. Carretero, E. Obrador, M. J. Anasagasti, et al., Clin. Exp. Metastas 17, 567 (1999).
L. de Leve and N. Kaplowitz, Sem. Liver Dis. 10, 251 (1990).
L. de Leve and N. Kaplowitz, Pharmacol. Ther. 52, 287 (1991).
C. Davies, J. Chem. Soc., 2093 (1938).
V. P. Vasil’ev, Thermodynamic Properties of Electrolyte Solutions (Vysshaya Shkola, Moscow, 1982), pp. 200, 313 [in Russian].
P. P. Korostelev, Preparation of Solutions for Chemical Analytical Work (Akad. Nauk SSSR, Moscow, 1962) [in Russian].
A. I. Lytkin, V. V. Chernikov, O. N. Krutova, and I. A. Skvortsov, J. Therm. Anal. Calorim. 130, 457 (2017).
D. G. Archer, J. Phys. Chem. Ref. Data 28, 1 (1999).
V. A. Borodin, V. P. Vasil’ev, and E. V. Kozlovskii, in Mathematic Problems of Chemical Thermodynamics (Nauka, Novosibirsk, 1985) [in Russian].
V. P. Vasil’ev, V. A. Borodin, and S. B. Kopnyshev, Zh. Fiz. Khim. 65, 55 (1991).
A. A. Gimadeev, E. V. Sagadeev, and V. P. Barabanov, Vestn. Kazan. Tekhnol. Univ., No. 2, 7 (2009).
Thermal Constants of Materials, Handbook, Ed. by V. P. Glushko (VINITI, Moscow, 1968), No. 3 [in Russian].
Funding
This work was performed at the Research Institute of Thermodynamics and Kinetics of Chemical Processes, Ivanovo State University of Chemical Technology, as part of State Task no. FZZW-2020-0009 (basic part).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lytkin, A.I., Chernikov, V.V., Krutova, O.N. et al. Standard Enthalpies of Formation of L-Glutathion and Products of Its Dissociation in Aqueous Solutions. Russ. J. Phys. Chem. 95, 1831–1834 (2021). https://doi.org/10.1134/S0036024421090156
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421090156