Abstract
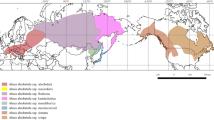
The Quaternary history of European nemoral forests in the east of their distribution may differ significantly from the typical dynamics of populations and ranges of most deciduous species in Western and Central Europe, characterized by survival in the Mediterranean refugia in glacial phases and recolonization in the interglacials. This study focuses on the phylogeography of the small-leaved lime Tilia cordata Mill. s. s. (Malvaceae) and related taxa in Eastern Europe, the Urals, Siberia, and the Crimea. The variability of five chloroplast DNA fragments (CDt, HKt, DT, K1K2, and psbJ–petA) was studied in 29 populations using restriction analysis and sequencing. The deep divergence of the six identified haplotypes and poorly supported topology of the phylogenetic tree presumably correspond to the long existence of T. cordata in several areas of the western Palaearctic isolated from each other. The two haplotypes dominate on the Russian Plain, in the Urals, and in Siberia. Their comparison with the data of other authors shows their absence in Western and Central Europe. Several haplotypes found in western Russia, in Belarus, and in Western Ukraine correspond to haplotypes previously identified in Central Europe and the Carpathians. We assume that such a distribution of chloroplast DNA variability is due to the preservation of lime in the refugia in the east of the range during one or several of the last glacial intervals and recolonization in the Late Glacial on the Russian Plain. Comparison of the results of the study of the variability of chloroplast DNA in T. cordata s. l. and the results of the study of nuclear microsatellites suggest that the species that lived in the Urals and the Russian Plain in the Pleistocene is a Siberian lime (T. sibirica), later displaced by the European small-leaved lime and now occurring only in Southern Siberia.



Similar content being viewed by others
REFERENCES
Hewitt, G.M., Some genetic consequences of ice ages, and their role in divergence and speciation, Biol. J. Linn. Soc., 1996, vol. 58, no. 3, pp. 247—276. https://doi.org/10.1111/j.1095-8312.1996.tb01434.x
Petit, R.J., Csaikl, U.M., Bordacs, S., et al., Chloroplast DNA variation in European white oaks—phylogeography and patterns of diversity based on data from over 2600 populations, For. Ecol. Manag., 2002, vol. 156, nos. 1—3, pp. 5—26. https://doi.org/10.1016/S0378-1127(01)00645-4
Heuertz, M., Carnevale, S., Fineschi, S., et al., Chloroplast DNA phylogeography of European ashes, Fraxinus sp. (Oleaceae): roles of hybridization and life history traits, Mol. Ecol., 2006, vol. 15, no. 8, pp. 2131—2140. https://doi.org/10.1111/j.1365-294X.2006.02897.x
Giesecke, T. and Brewer, S., Notes on the postglacial spread of abundant European tree taxa, Veget. Hist. Archaeobot., 2018, vol. 27, no. 2, pp. 337—349. https://doi.org/10.1007/s00334-017-0640-0
Magri, D., Vendramin, G.G., Comps, B., et al., A new scenario for the Quaternary history of European beech populations: palaeobotanical evidence and genetic consequences, New Phytol., 2006, vol. 171, no. 1, pp. 199—221. https://doi.org/10.1111/j.1469-8137.2006.01740.x
Sannikov, S.N., Petrova, I.V., Dymshakova, O.S., and Cherepanova, O.E., Genetic and phenotypic differentiation of Calluna vulgaris (L.) Hull. in Pritobolie and Europe, Russ. J. Genet., 2014, vol. 50, no. 9, pp. 925—933. https://doi.org/10.1134/S1022795414090129
Mandak, B., Havrdova, A., Krak, K., et al., Recent similarity in distribution ranges does not mean a similar postglacial history: a phylogeographical study of the boreal tree species Alnus incana based on microsatellite and chloroplast DNA variation, New Phytol., 2016, vol. 210, no. 4, pp. 1395—1407. https://doi.org/10.1111/nph.13848
Pyhäjärvi, T., Salmela, M.J., and Savolainen, O., Colonization routes of Pinus sylvestris inferred from distribution of mitochondrial DNA variation, Tree Genet. Genom., 2008, vol. 4, no. 2, pp. 247—254. https://doi.org/10.1007/s11295-007-0105-1
Semerikov, V.L., Semerikova, S.A., Putintseva, Y.A., et al., Colonization history of Scots pine in Eastern Europe and North Asia based on mitochondrial DNA variation, Tree Genet. Genom., 2018, vol. 14, no. 1. https://doi.org/10.1007/s11295-017-1222-0
Volkova, P.A., Schanzer, I.A., Soubani, E., et al., Phylogeography of the European rock rose Helianthemum nummularium s. l. (Cistaceae): western richness and eastern poverty, Plant Syst. Evol., 2016, vol. 302, no. 7, pp. 781—794. https://doi.org/10.1007/s00606-016-1299-1
Fineschi, S., Salvini, D., Taurchini, D., et al., Chloroplast DNA variation of Tilia cordata (Tiliaceae), Canad. J. For. Res., 2003, vol. 33, no. 12, pp. 2503—2508. https://doi.org/10.1139/X03-179
Havrdova, A., Douda, J., Krak, K., et al., Higher genetic diversity in recolonized areas than in refugia of Alnus glutinosa triggered by continent-wide lineage admixture, Mol. Ecol., 2015, vol. 24, no. 8, pp. 4759—4777. https://doi.org/10.1111/mec.13348
Flora SSSR (Flora of the Soviet Union), Moscow: Akad. Nauk SSSR, 1949, vol. 15.
Vasil’ev, I.V. and Svyazeva, O.A., Family Tiliaceae Juss., in Arealy derev’ev i kustarnikov SSSR (Distribution Ranges of Trees and Shrubs of the Soviet Union), Leningrad: Nauka, 1986, vol. 3, pp. 85—89.
Rysin, L.P., Lipovye lesa Russkoy ravniny (Lime Forests of the Russian Plain), Moscow: KMK, 2014.
Naumenko, N.I., The island location of Tilia cordata Mill. in the forest—steppe of the Tobol—Ishim interfluve: on the 45th anniversary of P.L. Gorchakovsky’s work about the West Siberian wing of small-leaved lime, Tilia cordata Mill. geographic range, Vestn. Udmurt. Univ., 2009, no. 2, Ser. 6, pp. 49—60.
Khlonov, Yu.P., Lipy i lipnyaki Zapadnoy Sibiri (Limes and Basswoods in Western Siberia), Novosibirsk: Sib. Otd. Akad. Nauk SSSR, 1965.
Logan, S.A., Chytry, M., and Wolff, K., Genetic diversity and demographic history of the Siberian lime (Tilia sibirica), Perspect. Plant Ecol. Evol. Syst., 2018, vol. 33, pp. 9—17. https://doi.org/10.1016/j.ppees.2018.04.005
Pigott, C.D., Lime-Trees and Basswoods: A Biological Monograph of the Genus Tilia, New York: Cambridge Univ. Press, 2012.
Phuekvilai, P., Relicts, refugia, and reticulation: a study of population history, hybrids and phylogeny in the long-lived flowering tree genus Tilia. Thesis Doctor of Philosophy, Newcastle Univ., 2014.
Grichuk, V.P., Istoriya flory i rastitel’nosti russkoi ravniny v pleistotsene (History of Flora and Vegetation of the Russian Plain in the Pleistocene), Moscow: Nauka, 1989.
Kulikov, P.V., Konspekt flory Chelyabinskoi oblasti (sosudistye rasteniya) (Synopsis of the Flora of the Chelyabinsk Region (Vascular Plants)), Yekaterinburg: Geotur, 2005.
Evolyutsiya ekosistem Evropy pri perekhode ot pleistotsena k golotsenu (24–8 tys. l. n.) (Evolution of European Ecosystems during Pleistocene—Holocene Transition (24—8 Thousand Years Ago)), van Kolfschoten, T. and Markova, A.K., Eds., Moscow: KMK, 2008.
Markova, A.K., Simakova, A.N., and Puzachenko, A.Y., Ecosystems of Eastern Europe at the time of maximum cooling of the Valdai glaciation (24—18 kyr BP) inferred from data on plant communities and mammal assemblages, Quat. Int., 2009, vol. 201, pp. 53—59. https://doi.org/10.1016/j.quaint.2008.05.020
Devey, M.E., Bell, J.C., Smith, D.N., et al., A genetic linkage map for Pinus radiata based on RFLP, RAPD and microsatellite markers, Theor. Appl. Genet., 1996, vol. 92, no. 6, pp. 673—679. https://doi.org/10.1007/BF00226088
Demesure, B., Sodzi, N., and Petit, R.J., A set of universal primers for amplification of polymorphic noncoding regions of mitochondrial and chloroplast DNA in plants, Mol. Ecol., 1995, vol. 4, no. 1, pp. 129—131. https://doi.org/10.1111/j.1365-294X.1995.tb00201.x
Cai, J., Ma, P.F., Li, H.T., and Li, D.Z., Complete plastid genome sequencing of four Tilia species (Malvaceae): a comparative analysis and phylogenetic implications, PLoS One, 2015, vol. 10, no. 11. https://doi.org/10.1371/journal.pone.0142705
Rozen, S. and Skaletsky, H.J., Primer 3 on the WWW for general users and for biologist programmers, in Bioinformatics Methods and Protocols: Methods in Molecular Biology, Krawetz, S. and Misener, S., Eds., Totowa, NJ: Humana Press, 2000, pp. 365—386.
Shaw, J., Lickey, E.B., Schilling, E.E., and Small, R.L., Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III, Am. J. Bot., 2007, vol. 94, no. 3, pp. 275—288. https://doi.org/10.3732/ajb.94.3.275
Hall, T.A., BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows95/98/NT, Nucleic Acids Symp. Ser., 1999, vol. 41, pp. 95—98.
Swofford, D.L., PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods): Version 4.0, Sunderland, Massachusetts: Sinauer Associates, 2002.
Ronquist, F. and Huelsenbeck, J.P., MrBAYES 3: Bayesian phylogenetic inference under mixed models, Bioinformatics, 2003, vol. 19, no. 12, pp. 1572—1574. https://doi.org/10.1093/bioinformatics/btg180
Bandelt, H.J., Forster, P., and Röhl, A., Median-joining networks for inferring intraspecific phylogenies, Mol. Biol. Evol., 1999, vol. 16, no. 1, pp. 37—48. https://doi.org/10.1093/oxfordjournals.molbev.a026036
McCarthy, D.M. and Mason-Gamer, R.J., Chloroplast DNA-based phylogeography of Tilia americana (Malvaceae), Syst. Bot., 2016, vol. 41, no. 4, pp. 865—880. https://doi.org/10.1600/036364416X693964
Semerikov, V.L., Semerikova, S.A., Polezhaeva, M.A., et al., Southern montane populations did not contribute to the recolonization of West Siberian plain by Siberian larch (Larix sibirica): a range-wide analysis of cytoplasmic markers, Mol. Ecol., 2013, vol. 22, no. 19, pp. 4958—4971. https://doi.org/10.1111/mec.12433
Semerikov, V.L., Semerikova, S.A., Putintseva, Y.A., et al., Mitochondrial DNA in Siberian conifers indicates multiple post-glacial colonization centers, Can. J. For. Res., 2019, vol. 49, no. 8, pp. 875—883. https://doi.org/10.1139/cjfr-2018-0498
Currat, M., Ruedi, M., Petit, R.J., and Excoffier, L., The hidden side of invasions: massive introgression by local genes, Evolution, 2008, vol. 62, no. 8, pp. 1908—1920. https://doi.org/10.1111/j.1558-5646.2008.00413.x
ACKNOWLEDGMENTS
We thank E.G. Filippov, L.I. Agafonov, Yu.Ya. Khrunyk, B.K. Gannibal, G.Yu. Konechnaya, E.V. Khantemirova, V.V. Kukarskikh, and E.V. Zinoviev for participation in the collection of lime samples. We also thank M.V. Modorov, A.I. Tsivilev, K.A. Panikovskaya, and N.V. Semerikov for their kind help with laboratory analyses. We also thank the anonymous referee for valuable comments.
Funding
This work was carried out within the framework of the State Contract of the Institute of Plant and Animal Ecology, Ural Branch, Russian Academy of Sciences.
This work was supported by the Russian Foundation for Basic Research, project no. 18-04-01061A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Bibov
Rights and permissions
About this article
Cite this article
Semerikova, S.A., Isakov, I.Y. & Semerikov, V.L. Chloroplast DNA Variation Shed Light on the History of Lime Tree (Tilia cordata s. l.) in the Eastern Part of the Range. Russ J Genet 56, 192–203 (2020). https://doi.org/10.1134/S1022795420020118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795420020118