Abstract
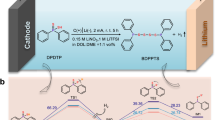
The prototypes of lithium batteries with organic electrode materials based on two lithium salts of polyhydroquionones containing azomethine groups and, for a comparison, materials based on the original Schiff base are developed and characterized. Poly[3,6-bis(iminomethylphenylene-1,2-diol)dilithium] and poly[3-(iminomethyl)-6-methylimino-N-(1-phenyl-4-diyl)benzene-1,2-dioldilithium] are synthesized and studied for the first time. For these structures, quantum chemical simulations are carried out for calculating the energy of the addition of lithium atoms which can proceed either to the nitrogen atom of the azomethine group or to the oxygen atom of the carbonyl group. It is shown experimentally that the best capacity and stability characteristics are demonstrated by the polymer poly[3,6-bis(iminomethylphenylene-1,2-diol)dilithium with the initial capacity of 140 mA h/g in the cycling interval of 0.7–4.1 V vs. Li+/Li, which makes it a promising cathodic material for lithium batteries.










Similar content being viewed by others
REFERENCES
Akitt, J.W., Kaye, F.W., Lee, B.E., and North, A.M., Conjugated polymeric Schiff bases. Thermally stable polymers with low electrical resistivity, Makromol. Chem., 1962, vol. 56, p. 195.
Li, X., Jiao, Y., and Li, S. The syntheses, properties and application of new conducting polymers, Eur. Polym. J., 1991, vol. 27, p. 1345.
Popov, Yu.A., Davydov, B.E., Kubasova, N.A., Kren-tsel’, B.A., and Konstantinov, I.I., Synthesis and properties of polymeric Schiff bases, Vysokomol.Soedinen., 1965, vol. 7, p. 835.
Cianga, I. and Ivanoiu, M., Synthesis of poly(Schiff-base)s by organometallic processes, Eur. Polym. J., 2006, vol. 42, p. 1922.
Kugler, T., Giguere, J.-B., Bourcet, F., and Toner, J., WO Patent 2018162890, 2018.
Kugler, T., Giguere, J.-B., Toner, J., and Bourcet, F., GB Patent 2560348, 2018.
Karushev, M.P., Belous, S.A., Lavrova, T.S., Chepurnaya, I.A., Timonov, A.M., and Kogan, S., WO Patent 2016044857, 2016.
Karushev, M.P., Belous, S.A., Lavrova, T.S., Chepurnaya, I.A., Timonov, A.M., and Kogan, S., WO Patent 2016028589, 2016.
Cheng, H., Sun, Y., Sun, Y., Pan, Q., and Sun, H., CN Patent 105261758, 2016.
Ye, H., Jiang, F., Li, H., Xu, Z., Yin, J., and Zhu, H., Facile synthesis of conjugated polymeric Schiff base as negative electrodes for lithium ion batteries, Electrochim. Acta, 2017, vol. 253, p. 319.
Sun, Y., Sun, Y., Pan, Q., Li, G., Han, B., Zeng, D., Zhang, Y., and Cheng, H., A hyperbranched conjugated Schiff base polymer network: a potential negative electrode for flexible thin film Batteries, Chem. Commun., 2016, vol. 52, p. 3000.
Zhuang, X., Zhang, F., Wu, D., and Feng, X., Graphene coupled Schiff-base porous polymers: Towards nitrogen-enriched porous carbon nanosheets with ultrahigh electrochemical capacity, Adv. Mater., 2014, vol. 26, p. 3081.
Fernandez, N., Sanchez-Fontecoba, P., Castillo-Martinez, E., Carretero-Gonzalez, J., Rojo, T., and Armand, M., Polymeric redox-active electrodes for sodium-ion batteries, ChemSusChem, 2018, vol. 11, p. 311.
Daigle, J.-C., Asakawa, Y., Hovington, P., Zaghib, K., Schiff base as additive for preventing gas evolution in Li4Ti5O12-based lithium-ion battery, ACS Appl. Mater. Interfaces, 2017, vol. 9(47), p. 41371.
Levchenko, N.F., Afanasiadi, L.Sh., and Bezuglyi, V.D., The influence of the nature of the radicals associated with the azomethine group on its polarographic activity, Zh. Obshch. Khim.,1967, vol. 37, p. 666.
Kitaev, Yu.P. and Troepol′skaya, T.V., Polarographic reduction of azomethine compounds, in Progress in Electrochemistry of Organic Compounds. Vol. 1, Frumkin, A.N. and Ershler, A.B., Eds., London: Plenum, 1971, p. 43.
Lopez-Herraiz, M., Castillo-Martınez, E., Carretero-Gonzalez, J., Carrasco, J., Rojo, T., and Armand, M., Oligomeric-Schiff bases as negative electrodes for sodium ion batteries: unveiling the nature of their active redox centers, Energy Environ. Sci., 2015, vol. 8, p. 3233.
Xiao, Z., Han, J., Xiao, J., Song, Q., Zhang, X., Kong, D., Yang, Q.-H., and Zhi, L., A facile and processable integration strategy towards Schiff-base polymer-derived carbonaceous materials with high lithium storage performance, Nanoscale, 2018, vol. 10, p. 10351.
Castillo-Martinez, E., Carretero-Gonzalez, J., and Armand, M., Polymeric Schiff bases as low-voltage redox centers for sodium-ion batteries, Angew. Chem., 2014, vol. 53, p. 5341.
Manecke, G., Wille, W.E., and Kossmehl, G., Preparation and properties of monomeric and polymeric Schiff bases derived from salicylaldehyde and 2,5-dihydroxyterephthalaldehyde. II. Electrical conductivity, Makromol. Chem., 1972, vol. 160, p. 111.
Mo, Y.-P., Liu, X.-H., Sun, B., Yan, H.-J., Wang, D., and Wan, L.-J., The intramolecular H-bonding effect on the growth and stability of Schiff-base surface covalent organic frameworks, Phys. Chem. Chem. Phys., 2017, vol. 19, p. 539.
Jiang, J., Dong, R.Y., and MacLachlan, M.J., Lyotropic liquid crystallinity in mixed-tautomer Schiff-base macrocycles, Chem. Commun., 2015, vol. 51, p. 16205.
Dunn, T.J., Ramogida, C.F., Simmonds, C., Paterson, A., Wong, E.W.Y., Chiang, L., Shimazaki, Y., and Storr, T., Non-innocent ligand behavior of a bimetallic Ni Schiff-base Complex containing a bridging catecholate, Inorg. Chem., 2011, vol. 50, p. 6746.
Akine, S., Sunaga, S., and Nabeshima, T., Multistep oligometal complexation of the macrocyclic tris(N2O2) hexaoxime ligand, Chem. – Eur. J., 2011, vol. 17, p. 6853.
Feltham, H.L.C., Clerac, R., Powell, A.K., and Brooker, S., A tetranuclear, macrocyclic 3d–4f complex showing single-molecule magnet behavior, Inorg. Chem., 2011, vol. 50, p. 4232.
Yamamura, M., Sasaki., Kyotani, M., Orita, H., and Nabeshima, T., Self-assembled nanostructures of tailored multi-metal complexes and morphology control by counter-anion exchange, Chem. – Eur. J., 2010, vol. 16, p. 10638.
Jiang, J. and MacLachlan, M.J., Unsymmetrical triangular Schiff base macrocycles with cone conformations, Org. Lett., 2010, vol. 12, p. 1020.
Gallant, A.J., Yun, M., Sauer, M., Yeung, C.S., and MacLachlan, M.J., Tautomerization in naphthalenediimines: A keto-enamine Schiff base macrocycle, Org. Lett., 2005, vol. 7, p. 4827.
Gallant, A.J. and MacLachlan, M.J., Ion-induced tubular assembly of conjugated Schiff-basemacrocycles, Angew. Chem. Int. Ed., 2003, vol. 42, p. 5307.
Akine, S., Taniguchi, T., and Nabeshima, T., Helical metallohost—guest complexes, J. Am. Chem. Soc., 2006, vol. 128, p.15765.
Perdew, P., Burke, K., and Ernzerhof, M., Generalized gradient approximation made simple, Phys. Rev. Lett. 1996, vol. 77, p. 3865.
Stevens, W.J., Basch, H., and Krauss, M.J., Valence basis set for transition metals (available Li–Rn) with corresponding ECPs, J. Chem. Phys., 1984, vol. 81, p. 6026.
Laikov, D.N., Fast evaluation of density functional exchange-correlation terms using the expansion of the electron density in auxiliary basis sets, Chem. Phys. Lett., 1997, vol. 281, p. 151.
ACKNOWLEDGMENTS
The quantum chemical simulations were carried out with the use of computers of the Joint Supercomputer Center of the Russian Academy of Sciences. We are grateful to S.Ya. Gadomskii for his help in the synthesis of (benzylidene)-p-phenylendiamine.
Funding
This study was carried out in accordance with the State Project no. 0089-2019-0010 (Institute of Problems of Chemical Physics, Russian Academy of Sciences) and was also supported by the Russian Scientific Foundation (grant no. 16-13-00111 (Skolkovo Institute of Science and Technology).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTERESTS
The authors declare the absence of any conflict of interests.
ADDITIONAL INFORMATION
ORCID ID 0000-0001-8819-8960 Shestakov A.F.
ORCID ID 0000-0001-9957-4140 Troshin P.A.
ORCID ID 0000-0002-3088-8165 Yarmolenko O.V.
Additional information
Translated by T. Safonova
Published on the basis of materials of the XIX All-Russian Conference “Electrochemistry of Organic Compounds” (EKHOS-2018) (with international participation), Novocherkassk, 2018.
Rights and permissions
About this article
Cite this article
Shestakov, A.F., Yakushchenko, I.K., Slesarenko, A.A. et al. Synthesis and Investigation of Dilithium Salts of Polyhydroquinones with Azomethine Groups as the Cathodes for Lithium Organic Batteries. Russ J Electrochem 56, 310–320 (2020). https://doi.org/10.1134/S1023193520040126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193520040126