Abstract
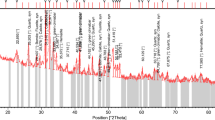
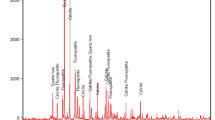
The research is aimed at removing uranium from low uranium content solutions using the ion flotation technique. The ion flotation process is an excellent technique for uranium separation from its solutions with low content or trace levels of uranium after optimizing carbonate–bicarbonate concentration for such solutions. It can also be applied to collecting uranium efficiently from all raffinates of the uranium separation or purification projects involving low-grade ore instead of other conventional long tedious methods such as ion exchange or solvent extraction, especially at low U levels. In this study, cetyltrimethylammonium bromide was used as a collector. The factors that can affect the flotation process (uranium concentration, gas flow rate, concentration of collector, and flotation time) were studied, and the best conditions were chosen: uranium concentration 0.02 g/L, carbonate concentration 10 g/L, gas flow rate 52 cm3/min, collector concentration 5 × 10–4 M, ethanol concentration 0.2% v/v, and flotation time 40 min. Under these conditions, the uranium flotation percentage reached more than 99%. A sample representing a sandy carbonaceous rock of Allouga area, southwestern Sinai, was prepared for alkaline leaching of uranium because of the high content of the carbonate which will consume large amounts of acids. The results of the experiments have shown that the optimum Na2CO3/NaHCO3 ratio is 1/1 at a total concentration of 80 g/L and S/L = 1/2, with 4-h agitation at room temperature. Under these conditions, the uranium leaching efficiency reached 94.2%, and the leachability increased to 98.7% at 80°C. The produced carbonate alkaline uranium-bearing leachate was subjected to the flotation process. A simplified sketch for the uranium separation from the carbonate solutions with a cationic collector is presented.













Similar content being viewed by others
REFERENCES
Tavera, F.J., Escudero, R, Uribe, A., and Finch, J.A., Afinidad, 2000, vol. 490, p. 415.
Sebba, F., Nature, 1959, vol. 184, p. 1062.
Mizuike, A. and Hiraide, M., Pure Appl. Chem., 1982, vol. 54, p. 1566.
Hoseinian, S., Rezai, B., Safari, M., Deglon, D., and Kowsari, E., J. Environ. Manag., 2019, vol. 244, pp. 408–414.
Grieves, R.B. and Wilson, T.E., Nature, 1965, vol. 205, p. 1066.
Deliyanni, E.A., Kyzas, G.Z., and Matis, K.A., J. Mol. Liq., 2017, vol. 225, p. 260.
Salmani, M.H., Davoodi, M., Ehrampoush, M.H., Ghaneian, M.T., and Fallahzadah, M.H., Iran. J. Environ. Health Sci. Eng., 2013, vol. 10, p. 16.
Chirkst, D.E., Lobacheva, O.L., Berlinskii, I.V., and Sulimova, M.A., Russ. J. Appl. Chem., 2009, vol. 82, no. 8, pp. 1273−1276.
Drakontis, C.E. and Amin, S., Curr. Opin. Colloid Interface Sci., 2020, vol. 48, pp. 77–90.
Kai Jia, Yuxia Yi, Wuju Ma, Yijun Cao, Guosheng Li, Shiqiang Liu, Taojin Wang, and Nan An, Miner. Eng., 2022, vol. 176, ID 107338.
Alexandrova, L. and Grigorov, L., Int. J. Miner. Process., 1996, vol. 48, pp. 111–125.
Zouboulis, A.I., Matis, K.A., and Stalidis, G.A., Innovations in Flotation Technology, Mavros, P., and Matis, K.A., Eds., Dordrecht: Kluwer, 1992.
Riegel, M., Tokmachev, M., and Hoell, W.H., React. Funct. Polym., 2008, vol. 68, pp. 1072–1080.
Bhalara, P.D., Punetha, D., and Balasubramanian, K., J. Environ. Chem. Eng., 2014, vol. 2, pp. 1621–1634.
Zhang, M., Yuan, M., Zhang, M., Wang, M., Chen, J., Li, R., Qiu, L., Fenga, X., Hu, J., and Wu, G., Radiat. Phys. Chem., 2020, vol. 171, ID 108742.
Tan, K., Li, C., Liu, J., Qu, H., Xia, L., Hu, Y., and Li, Y., Hydrometallurgy, 2014, vol. 150, pp. 99–106.
Bullwinkel, E.P., US Atomic Energy Commission, RMO, 1954, vol. 2614.
Jacebelli-Turi, C., Barocas, A., and Salvetti, F., Gazz. Chim. Ital., 1963, vol. 93, p. 1493.
Barocas, A., Jacobelli-Turi, C., and Salvetti, F., J. Chromatogr., 1964, vol. 14, p. 291.
Jacobelli-Turi, C., Barocas, A., and Terenzi, S., Ind. Eng. Chem. Process Des. Develop., 1967, vol. 6, p. 161.
Kunin, R., Ion Exchange Resins, Wiley, 1971, pp. 190–197.
Narayan, K., Village, W., and Pick, R., US Patent, 4092399, 1978.
Lyaudet, G., Mazarin, C., and Vial, J., US Patent, 4256702, 1981.
Riegel, M., Tokmachev, M., and Hoell, W., React. Funct. Polym., 2008, vol. 68, pp. 1072–1080.
Merritt, R.C., Extractive Metallurgy of Uranium, Merritt, R C., Ed.; Colorado School of Mines Research Inst., 1971.
Clark, D.L., Hobart, D.E., and Neu, M.P., Chem. Rev., 1995, vol. 95, p. 25.
Gupta, C.K. and Singh, H., Uranium Resource Processing: Secondary Resources, Mumbai, India: Bhabha Atomic Research Centre, 2001.
Forward, F.A. and Halpern, J., J. Met., 1954, vol. 6, p. 1408.
Shapiro, L. and Brannock, N.W., Rapid Analysis of Silicate, Carbonate and Phosphate Rocks, US Geological Survey Bulletin, 1962, no. 1144A.
Mathew, K.J., Bürger, S., Ogt, S.V., Mason, P.M.E.M., and Narayanan, U.I., in Eighth Int. Conf. on Methods and Applications of Radioanalytical Chemistry (Marc VIII), Kailua-Kona, Hawaii, 2009, vol. 5.
Yang Hu, Chunguang Li, Jiang Liu, Huiqiong Qu, Liangshu Xia, and Yongmei Li, Hydrometallurgy, 2014, vol. 150, pp. 99–106.
Mahmoud, M.R. and Othman, S.H., Radiochim. Acta, 2018, vol. 106, no. 6, pp. 465–476.
Corrin, M.L. and Harkins, W.D., J. Am. Chem. Soc., 1947, vol. 69, p. 683.
Ralston, A. and Hoerr, C.W., J. Am. Chem. Soc., 1946, vol. 68, p. 2460.
ACKNOWLEDGMENTS
The author is grateful to Prof. Dr. Kamal Abd Elbaki Ali Rabee, Nuclear Materials Authority (NMA), for his continuous and constructive assistance and for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that she has no conflict of interest.
Rights and permissions
About this article
Cite this article
Dayem, S.M.A.E. Studies for Ultimate Uranium Separation from Its Low-Content Carbonate Leachate Solutions by Ion Flotation. Radiochemistry 64, 193–202 (2022). https://doi.org/10.1134/S1066362222020116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362222020116