Abstract
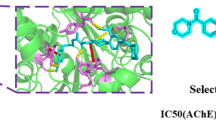
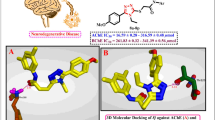
A new series of the anti-inflammatory drug ketoprofen derivatives bearing aryl chalcone-amide congeners were synthesized. The structures of the synthesized compounds were identified by the 1H NMR, 13C NMR, and EIMS spectroscopic methods. The inhibitory activity of the synthesized compounds on cholinesterase enzymes was investigated. Biological results revealed that five compounds displayed moderate activities against acetylcholinesterase (AChE) with IC50 values below 10 μM. Among the tested compounds, (BTPhP) was found to be the most potent against AChE (IC50 0.98 ± 0.02 μM), while the chalcone-amide analogues (MeOPh), (HydPh), (FPh), and (ChPh) exhibited moderate activities with IC50 values ranged between 5.19–9.61 μM. Molecular docking study showed that compound (BTPhP) could combine with the active site of acetylcholinesterase by the π–π between the ketoprofen phenyl groups is embedded in a cavity surrounded by two aromatic residues of Tyr334 and Trp279. The present results strongly suggest that the para-position of the D-ring should be a preferred modification site for further structural optimization design. Thus, compound (BTPhP) emerged as a promising lead for the development of new acetylcholinesterase inhibitor agent. The preliminary quantum structure-activity relationship (QSAR) among the newly synthesized congeners was obtained by Genetic Function Approximation (GFA).



Similar content being viewed by others
REFERENCES
Thies, W. and Bleile, L., Alzheimers Dement., 2013, vol. 9, pp. 208–245. https://doi.org/10.1016/j.jalz.2013.02.003
Prince, M., Ali, G.-C. Guerchet, M., Prina, M., Albanese, E., and Wu, T.-T., Alzheimer’s Res. Ther., 2016, vol. 8, pp. 23–35. https://doi.org/10.1186/s13195-016-0188-8
Hample, H., Mesuam, M.-M., Cuello, A.C., Farlow, M.R., Giacobini, E., Grossberg, G.T., Khachaturian, A.S., Vergallo, A., Cavedo, E., Snyder, P.J., and Khachaturian, Z.S., Brain, 2018, vol. 141, pp. 1917–1933. https://doi.org/10.1093/brain/awy132
Anand, P., Singh, B., and Singh, N., Bioorg. Med. Chem., 2012, vol. 20, pp. 1175–1180. https://doi.org/10.1016/j.bmc.2011.12.042
Schuster, D., Spetea, M., Music, M., Rief, S., Fink, M., Kirchmair, J., Schutz, J., Wolber, G., Langer, T., Stuppner, H., Schmidhammer, H., and Rollinger, J.M., Bioorg. Med. Chem., 2010, vol. 18, pp. 5071–5080. https://doi.org/10.1016/j.bmc.2010.05.071
Coyle, J.T., Price, D.L., and De Long, M.R., Science, 1983, vol. 219, pp. 1184–1190. https://doi.org/10.1126/science.6338589
Francis, P.T., Palmer, A.M., Snape, M., and Wilcock, G.K., J. Neurol. Neurosurg. Psychiatry, 1999, vol. 66, pp. 137–147. https://doi.org/10.1136/jnnp.66.2.137
Lane, R.M., Potkin, S.G., and Enz, A., Int. J. Neuropsychopharmacol., 2006, vol. 9, pp. 101–124. https://doi.org/10.1017/S1461145705005833
Giacobini, E., Pharmacol. Res., 2004, vol. 50, pp. 433–440. https://doi.org/10.1016/j.phrs.2003.11.017
Cacabelos, R., Torrellas, C., Teijido, O., and Carril, J.C., Pharmacogenomics, 2016, vol. 17, pp. 1041–1074. https://doi.org/10.2217/pgs-2016-0031
Goyal, D., Kaur, A., and Goyal, B., ChemMedChem, 2018, vol. 13, pp. 1275–1299. https://doi.org/10.1002/cmdc.201800156
Benek, O., Soukup, O., Pasdiorova, M., Hroch, L., Sepsova, V., Jost, P., Hrabinova, M., and Jun, D., ChemMedChem, 2016, vol. 11, pp. 1264–1269. https://doi.org/10.1002/cmdc.201500383
Tumiatti, V., J. Med. Chem., 2001, vol. 44, pp. 105–109. https://doi.org/10.1021/jm000991r
Sangnoi, Y., Sakulkeo, O., Yuenyongsawad, S., Kanjana-opas, A., Ingkaninan, K., Plubrukarn, A., and Suwanborirux, K., Mar. Drugs, 2008, vol. 6, pp. 578–586. https://doi.org/10.3390/md20080029
Liu, H.-R., Huang, X.-Q., Lou, D.-H., Liu, X.-J., Liu, W.-K., and Wang, Q.-A., Bioorg. Med. Chem. Lett., 2014, vol. 24, pp. 4749–4753. https://doi.org/10.1016/j.bmcl.2014.07.087
Zhao, F.-C., Wu, Y., and Song, X.-J., Med. Sci. Monit., 2017, vol. 23, pp. 3311–3317. https://doi.org/10.12659/MSM.901842
Díaz-Rubio, L., Hernández-Martinez, R., Estolano-Cobián, A., Chávez-Velasco, D., Salazar-Aranda, R., de Torres, N.W., Rivero, I.A., García-González, V., Ramos, M.A., and Córdova-Guerrero, I., App. Sci. 2019, vol. 9, pp. 410–439. https://doi.org/10.3390/app9030410
Ismail, M.M., Kamel, M.M., Mohamed, L.W., Faggal, S.I., and Galal, M.A., Molecules, 2012, vol. 17, pp. 7217–7231. https://doi.org/10.3390/molecules17067217
de Souza, G.A., da Silva, S.J., Del Cistia, C.N., Pitasse-Santos, P., Pires, L.O., Passos, Y.M., Cordeiro, Y., Cardoso, C.M., Castro, R.N., Sant’Anna, C.M.R., and Kümmerle, A.E., J. Enzyme Inhib. Med. Chem., 2019, vol. 34, pp. 631–637. https://doi.org/10.1080/14756366.2019.1571270
Zhou, X., Wang, X.-B., Wang, T., and Kon, L.-Y., Bioorg. Med. Chem., 2008, vol. 16, pp. 8011–8021. https://doi.org/10.1016/j.bmc.2008.07.068
Sonmez, F., Kurt, B.Z., Gazioglu, I., Basile, L., Dag, A., Cappello, V., Ginex, T., Kucukislamoglu, M., and Guccione, S., J. Enzyme Inhib. Med. Chem., 2017, vol. 32, pp. 285–297. https://doi.org/10.1080/14756366.2016.1250753
Guzior, N., Wi ckowska, A., Panek, D., and Malawska, B., Curr. Med. Chem., 2013, vol. 22, pp. 373–404. https://doi.org/10.2174/0929867321666141106122628
Anand, P. and Singh, B., Arch. Pharm. Res., 2013, vol. 36, pp. 375–399. https://doi.org/10.1007/s12272-013-0036-3
McHardy, S.F., Wang, H.-Y.L., McCowen, S.V., and Valdez, M.C., Expert Opin. Ther. Pat., 2017, vol. 27, pp. 455–476. https://doi.org/10.1080/13543776.2017.1272571
Sharma, K., Mol. Med. Rep., 2019, vol. 20, pp. 1479–1487. https://doi.org/10.3892/mmr.2019.10374
Zhuang, C., Zhang, W., Sheng, C., Zhang, W., Xing, C., and Miao, Z., Chem. Rev., 2017, vol. 117, pp. 7762–7810. https://doi.org/10.1021/acs.chemrev.7b00020
Willker, W., Leibfritz, D., Kerssebaum, R., and Bermel, W., Mag. Res. Chem., 1993, vol. 31, pp. 287–292. https://doi.org/10.1002/mrc.1260310315
Ellman, G.L., Courtney, K.D., Andresjr, V., and Featherstone, R.M., Biochem. Pharmacol., 1961, vol. 7, pp. 88–90, IN1, 91–95. https://doi.org/10.1016/0006-2952(61)90145-9
Molecular Operating Environment (MOE), ver. 2015.10, Chemical Computing Group Inc., Montreal, QC, Canada, 2015.
Barril, X. and Morley, S.D., J. Med. Chem., 2005, vol. 48, 4432–4443. https://doi.org/10.1021/jm048972v
DMOL 3 User Guide, San Diego, CA, USA: Accelrys, Inc., 2003.
Rogers, D. and Hopfinger, A.J., J. Chem. Inf. Comput. Sci., 1994, vol. 34, pp. 854–866. https://doi.org/10.1021/ci00020a020
Bliss, C.I., Microbiol. Mol. Biol. Rev., 1956, vol. 20, pp. 243–258. https://doi.org/10.1128/br.20.4.243-258.1956
ACKNOWLEDGMENTS
The authors thank Department of Chemistry, University of Basrah for the technical facilities. This paper is dedicated to the soul of Dr. Suha Al-Mosawi who passed away recently.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving human participants performed by any of the authors and does not contain any studies involving animals performed by any of the author.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Additional information
Abbreviations: BTPhP, 2-(3-benzoylphenyl)-N-(4-(3-(4-tolyl)acryloyl)phenyl) propanamide; MePPh, 2-(3-benzoylphenyl)-N-(4-(3-(4-methoxyphenyl)acryloyl)phenyl)propanamide; HydPh, 2-(3-benzoylphenyl)-N-(4-(3-(4-hydroxyphenyl)acryloyl)phenyl)propanamide; FPh, 2-(3-benzoylphenyl)-N-(4-(3-(4-fluorophenyl)acryloyl)phenyl)propanamide; ChPh, 2-(3-benzoylphenyl)-N-(4-(3-(4-chlorophenyl)acryloyl)phenyl)propanamide.
Supplementary Information
Rights and permissions
About this article
Cite this article
Al-Mosawi, S.K., Al-Hazam, H.A., Abbas, A.F. et al. Synthesis and QSAR of Novel Ketoprofen–Chalcone-Amide Hybrides as Acetylcholinesterase (AChE) Inhibitors for Possible Treatment of Alzheimer Disease. Russ J Bioorg Chem 48, 801–808 (2022). https://doi.org/10.1134/S1068162022040045
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022040045