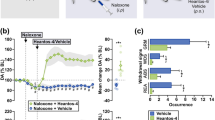
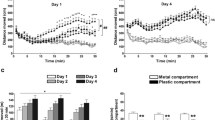
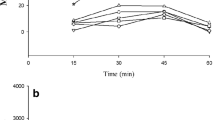
Abstract—Morphine is known to induce a long-term hyperlocomotor response due to increased dopamine release in the nucleus accumbens via the activation of dopaminergic neurons in the ventral tegmental area. It has been demonstrated that the low-affinity antagonist of NMDA receptors hemantane can alleviate ethanol-induced stimulation of behavior due to its effect on the dopamine- and noradrenergic systems. In the present study, we studied the effects of the aminoadamantane derivative hemantane on the changes in the balance of monoamines and their metabolites in brain structures of C57Bl/6 mice after acute morphine administration. Single i.p. administration of hemantane at a dose of 20 mg/kg did not affect spontaneous locomotor activity per se but alleviated behavior stimulated by s.c. morphine injection at a dose of 20 mg/kg (p < 0.05). In the ex vivo experiments, hemantane prevented a morphine-induced significant increase in the DOPAC/DA and 5‑HIAA/5-HT ratios in the striatum (both p < 0.05) and HVA/DA in the nucleus accumbens (p < 0.01) and promoted the recovery of the DOPAC/DA ratio to the control level in the hypothalamus. These data demonstrate the capability of hemantane to inhibit morphine-induced behavioral stimulation, probably via modulation of the dopaminergic and serotonergic systems.


Similar content being viewed by others
REFERENCES
Di Chiara, G. and Imperato, A., Proc. Natl. Acad. Sci. U.S.A., 1988, vol. 85, pp. 5274–5278.
Johnson, S.W. and North, R.A., J. Neurosci., 1992, vol. 12, pp. 483–488.
Maldonado, R., Saiardi, A., Valverde, O., Samad, T.A., Roques, B.P., and Borrelli, E., Nature, 1997, vol. 388, pp. 586–589.
Longoni, R., Spina, L., and Di Chiara, G., Psychopharmacology, 1987, vol. 93, pp. 401–402.
Kalivas, P.W., Widerlow, E., Stanley, D., Breese, G., and Prange, A.J., J. Pharmacol. Exp. Ther., 1983, vol. 227, pp. 229–237.
Serrano, A., Aguilar, M.A., Manzanedo, C., Rodriguez-Arias, M., and Minarro, J., Prog. Neuropsychopharmacol. Biol. Psychiatry, 2002, vol. 26, pp. 1263–1271.
Becker, A., Grecksch, G., Kraus, J., Peters, B., Schroeder, H., Schulz, S., and Hollt, V., Naunyn Schmiedebergs Arch. Pharmacol., 2001, vol. 363, pp. 562–568.
Borgkvist, A., Usiello, A., Greengard, P., and Fisone, G., Neuropsychopharmacology, 2007, vol. 32, no. 9, pp. 1995–2003.
Urs, N.M., Daigle, T.L., and Caron, M.G., Neuropsychopharmacology, 2011, vol. 36, no. 3, pp. 551–558.
Carroll, B.J. and Sharp, P.T., Br. J. Pharmacol., 1972, vol. 46 P, pp. 124–139.
Kitanaka, N., Kitanaka, J., and Takemura, M., Neurochem. Res., 2006, vol. 31, pp. 829–837.
Liu, X.S., Hou, Y., Yan, T.L., Guo, Y.Y., Han, W., Guan, F.L., Chen, T., and Li, T., CNS Neurosci. Ther., 2014, vol. 20, no. 9, pp. 823–829.
Katunina, E.A., Petrukhova, A.V., Avakyan, G.N., Val’dman, E.A., Nerobkova, L.N., Voronina, T.A., and Sayadyan, Kh.S., Zhurn. Nevrol. Psikhiatr.im.S.S. Korsakova, 2008, vol. 108, no. 6, pp. 24–27.
Nepoklonov, A.V., Kapitsa, I.G., Ivanova, E.A., Voronina, T.A., and Val’dman, E.A., Eksperim. Klin. Farmakol., 2012, vol. 75, no. 11, pp. 3–6.
Kolik, L.G., Nadorova, A.V., and Seredenin, S.B., Bull. Experim. Biol. Med., 2017, vol. 164, no. 2, pp. 152–157.
Kolik, L.G. and Konstantinopol’skii, M.A., Bull. Experim. Biol. Med., 2019, vol. 166, no. 6, pp. 739–743.
Val’dman, E.A., Voronina, T.A., Aksenova, L.N., Buneeva, O.A., and Medvedev, A.E., Eksperim. Klin. Farmakol., 2003, vol. 66, no. 5, pp. 3–5.
Abaimov, D.A., Zimin, I.A., and Kovalev, G.I., Eksperim. Klin. Farmakol., 2008, vol. 71, no. 1, pp. 18–21.
Abaimov, D.A., Zimin, I.A., Kudrin, V.S., and Kovalev, G.I., Eksperim. Klin. Farmakol., 2009, vol. 72, no. 1, pp. 64–67.
Elshanskaya, M.V., Sobolevskii, A.I., Khodorov, B.I., and Val’dman, E.A., Eksperim. Klin. Farmakol., 2001, vol. 64, no. 1, pp. 18–21.
Mori, T., Ito, S., Narita, M., Suzuki, T., and Sawaguchi, T., J. Pharmacol. Sci., 2004, vol. 96, pp. 450–458.
Kudrin, V.S., Nadorova, A.V., Narkevich, V.B., and Kolik, L.G., Neurochem. J., 2018, vol. 12, no. 1, pp. 64–70.
Melon, L.C. and Boehm, S.L., Alcohol Clin. Exp. Res., 2011, vol. 35, no. 7, pp. 1351–1360.
Suzuki, T., Maeda, J., Funada, M., and Misawa, M., Neurosci. Lett., 1995, vol. 187, no. 1, pp. 45–48.
Andyarzhanova, E.A., Val’dman, E.A., Kudrin, V.S., Raevskii, K.S., and Voronina, T.A., Eksperim. Klin. Farmakol., 2001, vol. 64, no. 6, pp. 13–16.
Kovalev, G.I., Rodionov, A.P., Petrenko, E.S., and Zolotarev, Yu.A., Eksperim. Klin. Farmakol., 2003, vol. 66, no. 3, pp. 50–52.
Desole, M.S., Esposito, G., Fresu, L., Migheli, R., Enrico, P., Mura, M.A., De Natale, G., Miele, E., and Miele, M., Brain Res., 1996, vol. 723, pp. 154–161.
Martin, G., Przewlocki, R., and Siggins, G.R., J. Pharmacol. Exp. Ther., 1999, vol. 288, pp. 30–35.
Guo, M., Xu, N.J., Li, Y.T., Yang, J.Y., Wu, C.F., and Pei, G., Neurosci. Lett., 2005, vol. 381, pp. 12–15.
Giacchino, J.L. and Henriksen, S.J., Prog. Neuropsychopharmacol. Biol. Psychiatry, 1998, vol. 22, pp. 1157–1178.
Liu, J., Nickolenko, J., and Sharp, F.R., Proc. Natl. Acad. Sci. U.S.A., 1994, vol. 91, pp. 8537–8541.
Pulvirenti, L., Swerdlow, N.R., and Koob, G.F., Pharmacol. Biochem. Behav., 1991, vol. 40, no. 4, pp. 841–845.
Funding
This study was performed according to theme no. 0521-2019-0006.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare no conflict of interest.
Ethical approval. All experiments with animals were performed in accordance with the protocol approved by the Biomedical Ethical Commission of the Zakusov Research Institute of Pharmacology.
Rights and permissions
About this article
Cite this article
Kolik, L.G., Nadorova, A.V., Narkevich, V.B. et al. Hemantane a Derivative of Aminoadamantane Alleviates Morphine-Induced Hyperlocomotion via Modulation of Activity of the Dopaminergic and Serotonergic Systems. Neurochem. J. 14, 55–63 (2020). https://doi.org/10.1134/S1819712420010134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1819712420010134