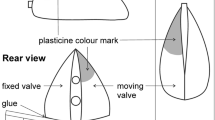
Abstract
Typical characteristics of the behavioral reactions of the Mediterranean mussel Mytilus galloprovicialis Lam, 1819 in its natural habitat of the Black Sea have been investigated using the originally developed automated biomonitoring complex of the aquatic environment. The valve movements exhibit a pronounced, clear solar diurnal rhythm, with maximum valve opening amplitude at night and minimum in the daytime at ambient natural environmental conditions throughout the year. Two types of valve movements are defined in the diurnal rhythm of the activity of the mussels inhabiting the Black Sea. Mollusks react acutely to abrupt fluctuations in the physical factors of the environment by closing the valves instantly for a short time, which is a manifestation of protective reflexes. The manifestation of stress signs is observed in behavioral reactions to an abnormal acute decrease in water temperature and prolonged exposure to a high water temperature.










Similar content being viewed by others
REFERENCES
Anestis, A., Lazou, A., Portner, H.O., and Michaelidis, B., Behavioral, metabolic, and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature, Am. J. Physiol. Regul. Integr. Comp. Physiol., 2007, vol. 293, pp. R911–R921. https://doi.org/10.1152/ajpregu.00124.2007
Barnes, G.E., The behaviour of Anodonta cygnea L., and its neurophysiological basis, J. Exp. Biol., 1955, vol. 32, pp. 158–174.
Borcherding, J., Ten years of practical experience with the Dreissena-monitor, a biological early warning system for continuous water quality monitoring, Hydrobiologia, 2006, vol. 556, pp. 417–426. https://doi.org/10.1007/s10750-005-1203-4
Comeau, L.A., Babarro, J.M.F., Longa, A., and Padin, X.A., Valve-gaping behavior of raft-cultivated mussels in the Ría de Arousa, Spain, Aquacult. Rep., 2018, vol. 9, pp. 68–73. https://doi.org/10.1016/j.aqrep.2017.12.005
Connor, K.M. and Robles, C.D., Within-site variation of growth rates and terminal sizes in Mytilus californianus along wave exposure and tidal gradients, Biol. Bull., 2015, vol. 228, pp. 39–51.
Curtis, T.M., Williamson, R., and Depledge, M.H., Simultaneous, long-term monitoring of valve and cardiac activity in the blue mussel Mytilus edulis exposed to copper, Mar. Biol., 2000, vol. 136, pp. 837–846.
Gnyubkin, V.F., The valve-movement model for the Mediterranean Mussel, Mytilus galloprovincialis Lamarck, 1819 (Bivalvia: Mytilidae), Russ. J. Mar. Biol., 2015, vol. 41, no. 1, pp. 40–51.
Gonzalez, J.G. and Yevich, P., Responses of an estuarine population of the blue mussel Mytilus edulis to heated water from a steam generating plant, Mar. Biol., 1976, vol. 34, no. 2, pp. 177–189. https://doi.org/10.1007/BF00390760
Gracey, A.Y. and Connor, K., Transcriptional and metabolomic characterization of spontaneous metabolic cycles in Mytilus californianus under subtidal conditions, Mar. Genomics, 2016, vol. 30, pp. 35–41. https://doi.org/10.1016/j.margen.2016.07.004
Hopkins, A.E., Galtsoff, P.S., and McMillin, H.C., Effects of pulp mill pollution on oysters [U.S.], Bur. Fish., 1931, vol. 47, no. 6, pp. 125–186.
Kholodov, V.I., Pirkova, A.V., and Ladygina, L.V., Vyrashchivanie midii i ustrits v Chernom more (Cultivation of Mussels and Oysters in the Black Sea), Sevastopol: Inst. Biol. Yuzh. Morei, 2010.
Kim, W.-S., Huh, H.-T., Je, J.-G., and Han, K.-N., Evidence of two-clock control of endogenous rhythm in the Washington clam, Saxidomus purpuratus, Mar. Biol., 2003, vol. 142, pp. 305–309.
Kramer, K.J.M. and Foekema, E.M., The “Musselmonitor®” as biological early warning system, in Biomonitors and Biomarkers as Indicators of Environmental Change 2, Environ. Sci. Res., 2001, vol. 56, p. 59–87.
Lesser, M.P., Bailey, M., Merselis, D., and Morrison, J.R., Physiological response of the blue mussel Mytilus edulis to differences in food and temperature in the Gulf of Maine, Comp. Biochem. Physiol., 2010, vol. 156, pp. 541–551.
Li, Y., Qin, J.G., Abbott, C.A., Li, X., and Benkendorff, K., Synergistic impacts of heat shock and spawning on the physiology and immune health of Crassostrea gigas: an explanation for summer mortality in Pacific oysters, Am. J. Regul. Integr. Comp. Physiol., 2007, vol. 293, no. 6, pp. 2353–2362.
Martella, T., Some factors influencing the byssus thread production in Mytilus edulis (Mollusca: Bivalvia) Linnaeus, 1758, Water, Air Soil Pollut., 1974, vol. 3, pp. 171–177.
Mat, A.M., Massabuau, J.-C., Ciret, P., and Tran, D., Looking for the clock mechanism responsible for circatidal behavior in the oyster Crassostrea gigas, Mar. Biol., 2014, vol. 161, no. 1, pp. 89–99.
Naylor, E., Chronobiology of Marine Organisms, Cambridge: Cambridge Univ. Press, 2010.
Newell, C.R., Wildish, D.J., and MacDonald, B.A., The effects of velocity and seston concentration on the exhalant siphon area, valve gape and filtration rate of the mussel Mytilus edulis, J. Exp. Mar. Biol. Ecol., 2001, vol. 262, pp. 91–111.
Ortmann, C. and Grieshaber, M., Energy metabolism and valve closure behaviour in the Asian clam Corbicula fluminea, J. Exp. Biol., 2003, vol. 206, pp. 4167–4178.
Riisgard, H.U., Egede, P.P., and Saavedrai, B., Feeding behaviour of the mussel, Mytilus edulis: new observations, with a minireview of current knowledge, J. Mar. Biol., 2011, vol. 2011, ID 312459.
Robson, A. and de Leaniz, C.G., Effect of anthropogenic feeding regimes on activity rhythms of laboratory mussels exposed to natural light, Hydrobiologia, 2010, vol. 655, pp. 197–204. https://doi.org/10.1007/s10750-010-0449-7
Ryabushko, V.I., Kozintsev, A.F., Makarchuk, T.L., and Shinkarenko, V.K., The content of heavy metals in the mussel Mytilus galloprovincialis from the Kazach’ya Bay (Black Sea), Morsk. Biotekh. Sist., 2002, no. 2, pp. 215–221.
Saurel, C., Gascoigne, J.C., Palmer, M.R., and Kaiser, M.J., In situ mussel feeding behavior in relation to multiple environmental factors: regulation through food concentration and tidal conditions, Am. Soc. Limnol. Oceanogr., 2007, vol. 52, no. 5, pp. 1919–1929.
Somero, G.N., Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living, Integr. Comp. Biol., 2002, vol. 42, no. 4, pp. 780–789.
Tran, D., Nadau, A., Durrieu, G., et al., Field chronobiology of a molluscan bivalve: how the moon and sun cycles interact to drive oyster activity rhythms, Chronobiol. Int., 2011, vol. 28, no. 4, pp. 307–317.
Tran, D., Sow, M., Camus, L., et al., In the darkness of the polar night, scallops keep on a steady rhythm, Sci. Rep., 2016, vol. 6, p. 32435. https://doi.org/10.1038/srep32435
Trusevich, V.V., Gaiskii, P.V., and Kuz’min, K.A., Automated biomonitoring of the aquatic environment using the responses of bivalves, Morsk. Gidrofiz. Zh., 2010, no. 3, pp. 75–83.
Trusevich, V.V., Gaiskii, P.V., Kuz’min, K.A., and Mishurov, V.Zh., Biomarkers of behavioral responses of the Black Sea mussel for automated monitoring of the aquatic environment, Sist. Kontr. Okruzh. Sredy, 2015, no. 1 (21), pp. 13–18.
de Zwaan, A. and Wijsman, T.C.M., A. Review: anaerobic metabolism in Bivalvia (Mollusca), Charact. Anaerobic Metab., 1976, vol. 56B, pp. 313–324.
Funding
This study was supported by the Russian Foundation for Basic Research and the city of Sevastopol, project no. 18-45-920061.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Martynova
Rights and permissions
About this article
Cite this article
Trusevich, V.V., Kuz’min, K.A., Mishurov, V.Z. et al. Features of Behavioral Responses of the Mediterranean Mussel in Its Natural Habitat of the Black Sea. Inland Water Biol 14, 10–19 (2021). https://doi.org/10.1134/S1995082921010132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082921010132