Abstract
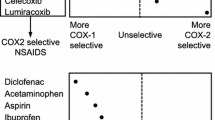
Selective COX-2 inhibition relative to COX-1 has consistently been demonstrated for meloxicam in various in vitro test systems. In human platelets ex vivo COX-1-dependent thromboxane formation is partially and dose dependently inhibited, however no significant inhibition of platelet aggregation has been observed with the recommended doses of 7.5 mg and 15 mg meloxicam daily. With once daily dosing, meloxicam has demonstrated efficacy in osteoarthritis, rheumatoid arthritis and ankylosing spondylitis. Meloxicam was granted its first marketing authorization in 1995 and is now available in more than 100 countries. Meloxicam has been studied in clinical trials involving more than 30 000 patients and is estimated to have been prescribed for more than 30 million patients worldwide to date. Clinically, meloxicam offers similar efficacy to recommended doses of well-established nonsteroid anti-inflammatory drugs (NSAIDs), including diclofenac, piroxicam and naproxen. Meloxicam distinguishes itself from established NSAIDs with a reduced risk of certain gastrointestinal adverse events. This has consistently been demonstrated in randomized clinical trials, large scale clinical outcome studies, pooled analyses and meta-analyses. Post-marketing experience is consistent with the safety profile established in these studies and analyses.
Similar content being viewed by others
REFERENCES
Carrabba, M., Paresce, E., Angelini, M., et al (1995). A comparison of the local tolerability, safety and efficacy of meloxicam and piroxicam suppositories in patients with osteoarthritis: a single-blind, randomized, multicentre study, Curr. Med. Res. Opin. 13 (6), 343–355.
Churchill, L., Graham, A. G., Shih, C. K., et al. (1996). Selective inhibition of human cyclo-oxygenase-2 by meloxicam, Inflammopharmacology 4, 125–135.
Degner, F., Sigmund, R. and Zeidler, H. (2000). Efficacy and tolerability of meloxicam in an observational, controlled cohort study in patients with rheumatic disease, Clin. Ther. 22 (4), 400–410.
De Meijer, A., Vollaard, H., de Metz, M., et al (1999). Meloxicam 15 mg/day spares platelet function in healthy volunteers, Clin. Pharmacol. Ther. 66, 425–430.
Dequeker, J., Hawkey, C., Kahan, A., et al (1998). Improvement in gastrointestinal tolerability of the selective cyclooxygenase (COX)-2 inhibitor, meloxicam, compared with piroxicam: results of the Safety and Efficacy Large-scale Evaluation of COX-inhibiting Therapies (SELECT) trial in osteoarthritis, Brit. J. Rheumatol. 37 (9), 946–951.
Distel, M., Mueller, C. and Bluhmki, E. (1996). Global analysis of gastrointestinal safety of a new NSAID, meloxicam, Inflammopharmacology 4, 71–81.
Dougados, M., Gueguen, A., Nakache, J. P., et al (1999). Ankylosing spondylitis: what is the optimumduration of a clinical study? A one year versus a 6 weeks non-steroidal anti-inflammatory drug trial, Rheumatology 38 (3), 235–244.
Engelhardt, G., Homma, D., Schlegel, K., et al (1995). Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-in' ammatory agent with favourable gastrointestinal tolerance, Inflamm. Res. 44, 423–433.
Furst, D., Hall, D., Roszko, P. J., et al (2000). Efficacy, Safety and Dose Response of Meloxicam up to 22.5 mg in the treatment of rheumatoid arthritis (RA): Results of a phase III double-blind, placebo controlled trial. ACR 64 th Annual Scientific Meeting, Philadelphia.
Ghozlan, P. R., Bernhardt, M., Velicitat, P., et al (1996). Tolerability of multiple administration of intramuscular meloxicam: a comparison with intramuscular piroxicamin patients with rheumatoid arthritis or osteoarthritis, Brit. J. Rheumatol. 35 (Suppl. 1), 51–55.
Goei The, H. S., Lund, B., Distel, M. R., et al (1997). A double-blind, randomized trial to compare meloxicam 15 mg with diclofenac 100 mg in the treatment of osteoarthritis of the knee, Osteoarthritis Cartilage 5 (4), 283–288.
Hawkey, C. J. (1999). COX-2 inhibitors, Lancet 353, 307–314.
Hawkey, C., Kahan, A., Steinbrück, K., et al. (1998). Gastrointestinal tolerability of meloxicam com-pared to diclofenac in osteoarthritis patients. International MELISSA Study Group. Meloxicam Large-scale International Study Safety Assessment, Brit. J. Rheumatol. 37 (9), 937–945.
Hosie, J., Distel, M. and Bluhmki, E. (1996). Meloxicam in osteoarthritis: a 6-month, double-blind comparison with diclofenac sodium, Brit. J. Rheumatol. 35 (Suppl. 1), 39–43.
Hosie, J., Distel, M. and Bluhmki, E. (1997). Efficacy and tolerabilityof meloxicamversus piroxicam in patients with osteoarthritisof the hip or knee: a six-month double-blindstudy, Clin. Drug Invest. 13 (4), 175–184.
Huskisson, E. C., Narjes, H. and Bluhmki, E. (1994). Efficacy and tolerance of meloxicam, a new NSAID, in daily oral doses of 15, 30 and 60 mg in comparison to 20 mg piroxicamin patients with rheumatoid arthritis, Scand. J. Rheumatol. S98, 115.
Lanes, S. F., Garcia Rodriguez, L. A. and Hwang, E. (2000). Baseline risk of gastrointestinal disorders among new users of meloxicam, ibuprofen, diclofenac, naproxen and indomethacin, Pharmacoepidemiol. Drug Safety 9, 113–117.
Lemmel, E. M., Bolten, W., Burgos-Vargas, R., et al. (1997). Efficacy and safety of meloxicam in patients with rheumatoid arthritis, J. Rheumatol. 24, 282–290.
Linden, B., Distel, M. and Bluhmki, E. (1996). A double-blind study to compare the efficacy and safety of meloxicam 15 mg with piroxicam 20 mg in patients with osteoarthritis of the hip, Brit. J. Rheumatol. 35 (Suppl. 1), 35–38.
Lund, B., Distel, M. and Bluhmki, E. (1998). A double-blind, randomized, placebo-controlledstudy of efficacy and tolerance of meloxicamtreatment in patients with osteoarthritis of the knee, Scand. J. Rheumatol. 27, 32–37.
Martin, R. M., Biswas, P. and Mann, R. D. (2000). The incidence of adverse events and risk factors for upper gastrointestinal disorders associated with meloxicamuse amongst 19087 patients in general practice in England: cohort study, Brit. J. Clin. Pharmacol. 50, 35–42.
Panara, M. R., Renda, G., Sciulli, M. G., et al (1999). Dose-dependent inhibition of platelet cyclooxygenase-1 and monocyte cyclooxygenase-2 by meloxicam in healthy subjects, J. Phar-macol. Exp. Ther. 290 (1), 276–280.
Patrignani, P., Panara, M. R., Sciulli, M. G., et al. (1997). Differential inhibition of human prostaglandinendoperoxide synthase-1 and-2 by nonsteroidal anti-inflammatory drugs, J. Physiol. Pharmacol. 48, 623–631.
Schoenfeld, P. (1999). Gastrointestinal safety profile of meloxicam: a meta-analysis and systematic review of randomized controlled trials, Amer. J. Med. 107 (Suppl. 6A), 48S–54S.
Silverstein, F. E., Faich, G. F., Goldstein, J. L., et al (2000). Gastrointestinal toxicity with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis, J. Amer. Med. Assn. 284, 1247–1255.
Stichtenoth, D. O., Wagner, B. and Frölich, J. C. (1997). Effects of meloxicam and indomethacin on cyclooxygenase pathways in healthy volunteers, J. Invest. Med. 45, 44–49.
Van Hecken, A., Schwartz, J. I., Depre, M., et al. (2000). Comparative inhibitory activity of rofecoxib (MK-0966), meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 vs. COX-1 in healthy volunteers, J. Clin. Pharmacol. 40, 1109–1120.
Warner, T., Giuliano, F., Vojnovic, I., et al. (1999). Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2are associated with human gastrointestinaltoxicity: A full in vitro analysis, Proc. Natl. Acad. Sci. USA 96, 7563–7568.
Wojtulewski, J. A., Schattenkirchner, M., Barcelo, P., et al. (1996). A six-month double-blind trial to compare the efficacy and safety of meloxicam7.5 mg daily and naproxen 750 mg daily in patients with rheumatoid arthritis, Brit. J. Rheumatol. 35 (Suppl. 1), 22–28.
Yocum, D., Fleischmann, R., Dalgin, P., et al. (2000). Efficacy and safety of meloxicam in the treatment of osteoarthritis, Arch. Intern. Med. 160, 2947–2954.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Degner, F., Richardson, B. Review of gastrointestinal tolerability and safety of Meloxicam. Inflammopharmacology 9, 71–80 (2001). https://doi.org/10.1163/156856001300248344
Issue Date:
DOI: https://doi.org/10.1163/156856001300248344